Curiously enough, indirect support for mRNA vaccines driving Covid infections and deaths is also suggested by implication by India’s example. Anyone who has followed the Covid story globally will recall the horrific images of bodies being washed ashore on the banks of the Ganga in April–June 2021, and crematoriums unable to cope with the piles of bodies awaiting the last Hindu rites of being put to flame on funeral pyres.
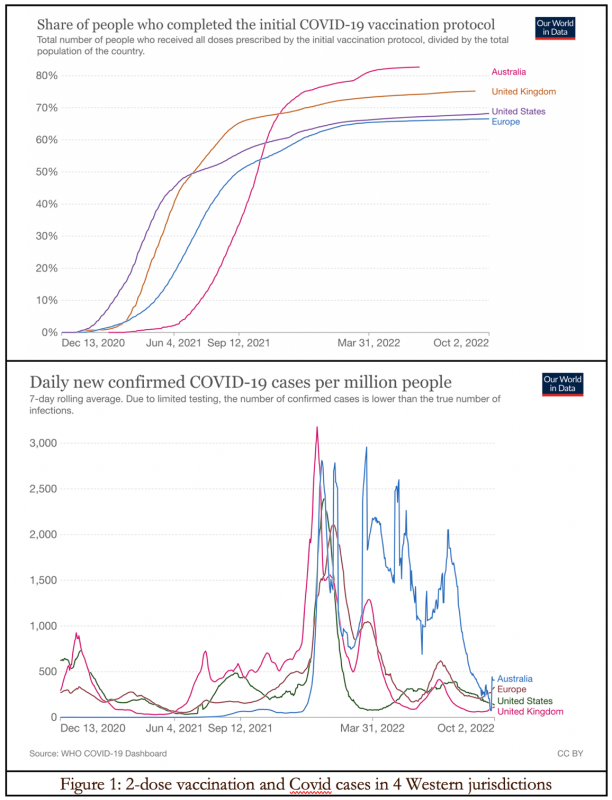
In Figure 1, we can see a lagged temporal correlation between vaccine rollout starting in late 2020 and early 2021, and a rise in Covid-19 infections from autumn 2021, in Australia, Europe, the UK, and the US. Had the vaccines proven 95 percent effective against infection and transmission in the real world, this should not have happened.
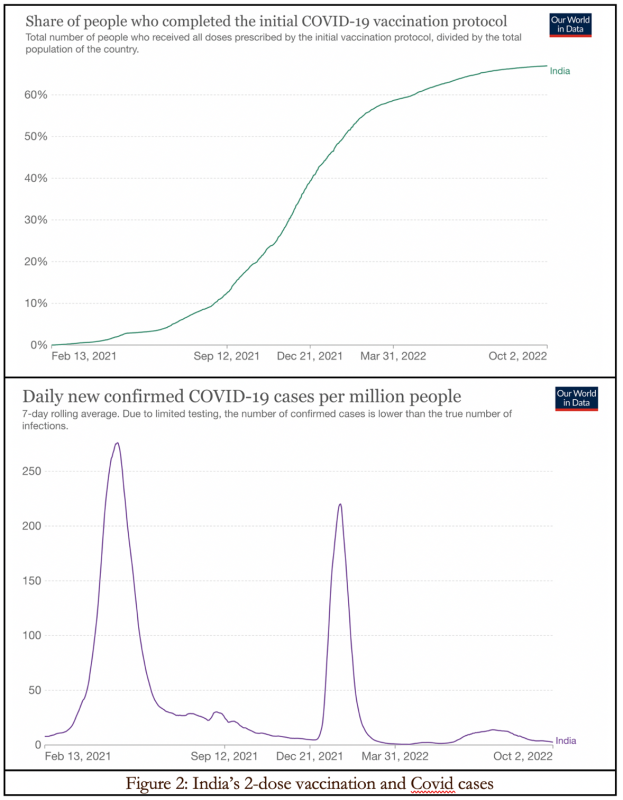
Figure 2 shows a three-month wave in Covid infections in India in April–June 2021 inclusive, followed by a two-month seasonal winter surge in January–February 2022. There is no correlation with the vaccine rollout.
As per Our World in Data, India’s daily new Covid death count rose above 1,000 for the first time on 16 April 2021, peaked at 4,190 on 23 May, and fell below 1,000 again on 1 July. It climbed back above 1,000 briefly with a subsequent ‘ripple’ with 1,127 deaths on 5 February 2022. India’s double vaccination coverage was only 1.1 percent on 16 April 2021, still under 3 percent on 23 May and 4.2 percent on 1 July. By February 2022 it had reached 50 percent.
How does this help with the notion that mRNA vaccines might be driving Covid cases and deaths in many Western countries? Because the main vaccine administered in India is a virus-vector type. The Indian government would not agree to demands by Pfizer and Moderna that their approvals elsewhere, based on trial results from overseas, were sufficient to be granted emergency use authorization in India with full legal indemnity.
India’s drug regulator, the Central Drugs Standard Control Organisation, said in February 2021 that its experts did not recommend the Pfizer vaccine because side effects reported abroad were still being investigated. It also said Pfizer had not proposed any plan to generate safety and immunogenicity data in India.
In contracts with several European and Latin American countries, Pfizer required the recipient states to acknowledge that long-term effects and efficacy of the vaccine are currently unknown and there may be adverse effects, contradicting both parts of the “safe and effective” mantra drilled into all public messaging.
Instead India administered two main types, neither of which used mRNA technology. Covishield, the AstraZeneca vaccine, is a viral vector vaccine that uses a weakened, non-replicating strain of chimpanzee cold virus (adenovirus) to carry genetic material of the spike protein of SARS-CoV-2 into human cells. This accounted for more than four-fifths of vaccines administered in India by April 2022.
The second was Covaxin that was developed by the local firm Bharat Biotech in partnership with the Indian Council of Medical Research. Covaxin contains an inactivated SARS-CoV-2 virus that has been disabled for replication. All of its 29 proteins are intact and provoke immunity of the host that is closer to the infection-induced natural immunity. Coming out of decades of research in adenovirus-based vaccines, it uses a tried and tested technology platform with an established safety profile that has been used by other vaccines like polio.
As I understand it, Pfizer-BioNTech and Moderna make mRNA vaccines. These give our cells instructions for how to make the spike (S) protein found in the SARS-CoV-2 virus that it uses to enter human cells. After vaccination, muscle cells begin making the S protein pieces which stimulate the body to create antibodies. If infected with SARS-CoV-2, the antibodies will fight the virus.
In a vector vaccine, material from the SARS-CoV-2 virus is placed in a modified version of a different virus (viral vector). The latter gives the cells instructions to make copies of the SARS-CoV-2 S protein. When the cells display the S proteins on their surfaces, the immune system responds by creating antibodies and defensive white blood cells. Upon infection with SARS-CoV-2, the antibodies will fight the virus.
According to a Danish study by Professor Christine Benn and colleagues reviewing randomized controlled trials (RCTs) of mRNA and adenovirus vector vaccines that was published in preprint a year ago, the adenovirus vector vaccines reduce all-cause mortality significantly, compared with the mRNA vaccines that demonstrate no benefit in terms of all-cause mortality. The relative risk of mRNA vaccines compared to the placebo group was 1.03 percent, and that of the adenovirus vector vaccines was 0.37 percent.
Recently Germany’s Die Welt became the first major mainstream publication to report on the allegations of fraud in Pfizer’s clinical trials, with participants who suffered adverse events being unblinded and removed and cover-ups of the death of Pfizer subjects. The New York Times has taken the European Commission to court over Commission President Ursula von der Leyen’s refusal to release the text messages she exchanged with Pfizer CEO Albert Bourla, in which she personally negotiated the purchase of up to 1.8 billion doses of the BioNTech/Pfizer vaccine. On 15 February Florida issued a health alert on mRNA Covid-19 vaccine safety.
When we add this to the long list of out of court settlements by Pfizer and some convictions, perhaps India did well to dodge this bullet!
In the US, Americans funded the development of Covid vaccines by Pfizer and Moderna, paid for the resulting vaccines, were mandated to take the jabs by governments and many private sector employers, but because of the grant of legal indemnity lost their right to hold commercial entities accountable for neglect and malfeasance.
No one has as yet explained why, if the manufacturers were so convinced that their vaccines were safe and effective, they required legal indemnity against vaccine injuries. Nor have supposedly progressive governments such as New Zealand’s, as well as conservative governments like Australia’s at the time, attempted to justify the logic behind privatizing the profits but socializing the risks.
Some US states like North Dakota and Arkansas are reportedly considering legislation (1) to prevent mandatory medical treatment if the product manufacturer cannot be held legally liable, or (2) to “make it criminal for pharmaceutical executives to knowingly hide, conceal, or withhold information regarding a medical product’s adverse effects if the product results in death or serious injury.” This would restore citizens’ rights against state-subsidized Big Pharma with its immense lobbying budgets, power, and successes.
Meanwhile India’s contra example poses some difficult questions for the drug regulators of countries like Australia. Why is it that local clinical trials were not required before granting emergency use authorization to these experimental vaccines with no established long-term safety profiles? Have they been captured by the very industry they are meant to regulate in order to protect public health?
Do they understand why there is public cynicism that regulators might have morphed from guardians of public health into vaccine enablers, accelerating the approval process by short-circuiting safety trials, yet being noticeably tardy in responding to safety signals and investigating vaccine injuries, as in the case of 24-year-old Amy Sedgwick whose tragic death was featured in The Weekend Australian on 25 March ?
In a poignant follow-up Adam Creighton, the paper’s US correspondent, pointed out that this story would have been disappeared from the Internet in 2021 owing to the censorship collusion between Big Pharma, Big Tech, and public health authorities.
Published under a Creative Commons Attribution 4.0 International License
For reprints, please set the canonical link back to the original Brownstone Institute Article and Author.









