“Judge, I was forced to take an experimental vaccine!”
Kaiser Permanente in CA is getting sued:
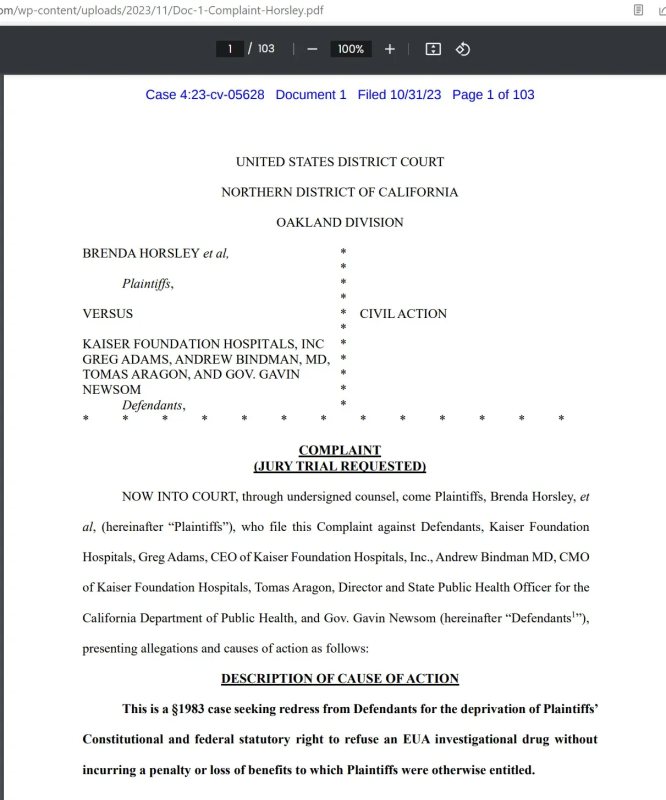

Here is another example – Roberts v Shriners. The case cites correct law conserning EUA (360bbb):


Then states that “FDA defines the drugs at issue as investigational:”


That is what FDA says about these drugs to the public, it is not how they are defined in law. The FDA also says they are “fully approved” now – a legal impossibility for EUA substances. The FDA also says they are “safe and effective,” and we know that’s a lie, too.
Note that I am not a lawyer, and I am not asking you to hire me for this or any other case in any capacity, and I gave absolutely zero financial or any other interest to point this out. However, why do these lawyers not read the law?
An EUA drug under PHE and current PREP Act declarations is NOT an investigational drug! It is a legal impossibility for it to be investigational or experimental by definition of its status as EUA.
Bailiwick News Katherine Watt wrote about Bridges v Methodist case here.
Note that the case was dismissed in June 12, 2021 order by USDJ Lynn N. Hughes quoted below (emphasis mine):
On April 1, 2021, Houston Methodist Hospital announced a policy requiring employees be vaccinated against COVID-19 by June 7, 2021, starting with the leadership and then inoculating the remaining workers, all at its expense.
Jennifer Bridges and 116 other employees sued to block the injection requirement and the terminations. She argued that Methodist is unlawfully forcing its employees to be injected with one of the currently available vaccines or be fired. The hospital has moved to dismiss this case.
Bridges dedicates the bulk of her pleadings to arguing that the currently available COVID-19 vaccines are experimental and dangerous. This claim is false, and it is also irrelevant. Bridges argues that, if she is fired for refusing to be injected with a vaccine, she will be wrongfully terminated. Vaccine safety and efficacy are not considered in adjudicating this issue.
Judge Hughes declared Bridges’ assertion that the ‘vaccines’ are experimental and dangerous to be “false.” Turns out the judge is technically correct about the experimental part, while being morally deplorable, of course. Note that the “dangerous” part was never allowed to be argued in court and this is by design of the Covid campaign and it’s illegal-legal structure.
This structure (aka “Covid kill box”) was designed carefully over the years to ensure that most professionals involved in it are either: fooled; or given plausible deniability to act as if they were fooled; or given lots of financial incentives to act as if they were fooled.
However, Bridges would have had a far better chance of getting to present the evidence of danger had she based her case on the applicable current law. It is false to assert that EUA vaccines are medical experiments, because they are not, as I will explain further in this article.
Before I do that, here is another example of widespread misunderstanding of the nature of the Covid military campaign – the ubiquitous in the “health freedom” crowd narrative of “dolts botching shit” (credit Sage Hana).
Here is one such example:
“Incompetent pharmas can’t mass produce anything!”


The claim therein is that for 3 whole years nobody in pharma manufacturing noticed that they are shipping poison laced with many other poisons, and that this is because utterly incompetent medical people just don’t know how to manufacture things to pharmaceutical grade standards. If only car companies made vaccines! My eyes are rolling so far back into my head, I am seeing the back of my skull and it’s not pretty.
Yeah… If only… If this hypothesis is correct, then why are all other drugs shipped seemingly within manufacturing tolerances? How come pharmas seem to be able to mass produce those things? And when they are not, AG of Texas is suing manufacturer for product adulteration? Why hasn’t fearless Ken Paxton filed this same lawsuit for Covid vaccines? Based on my knowledge of thousands of vials tested all over the world, not a single vial has been found in conformance with the allegedly “FDA-approved” product label. It would be a slam dunk. And the fines he could have recovered would be OMG, eyewatering!
Yet, he has filed a lawsuit v Pfizer for “deceptive marketing practices” and NOT manufacturing fraud. For some reason he is not suing Moderna, which engaged in the same deceptive marketing practices and media collusion. Note – I will write a separate post about Paxton’s lawsuit, which is better articulated than others, but commits the same fallacy with regard to the EUA. What is so special about Covid vaxxes? Think! Think harder….
Oh, I know.
Let’s look what “EUA” regulatory status means. Do EUA countermeasures have to adhere to the current Good Manufacturing Practices? Let’s consult US Code:


Looks like the answer is “no.”
But what do I know? Maybe we should ask Ford Motor Company to opine. Maybe a better group of people to consult is the FDA lawyers. Here’s what they say in their own “legal preparedness” [preparedness to break FD&C Act] powerpoint presentations:


We know we need to break the law to put this poison on the market, so, we need to make a parallel universe where the illegal things are called legal and we are good. Let’s call it EUA. Stroke of genius, I must admit.
On this page, they plainly state that “EUA use is not investigational, so IRB approval and informed consent are not required.”


And for avoidance of any doubt – clinical trials (as legally defined human experiment) do not exist for EUA:
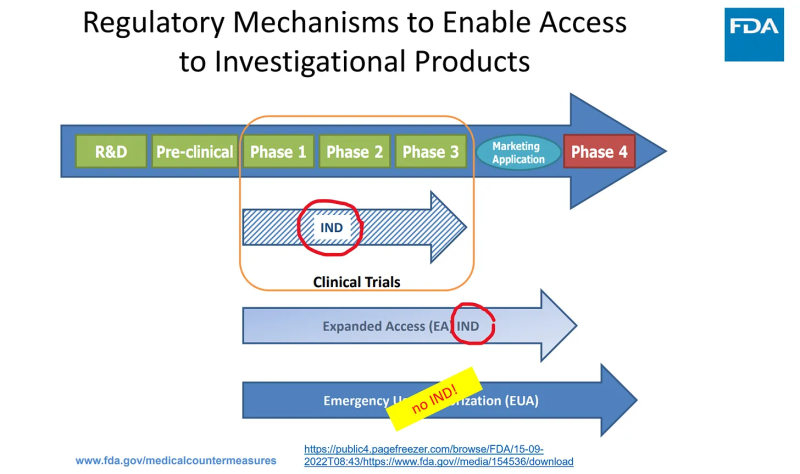

The FDA lawyers found a way to break the FD&C Act by creating a separate section in it (random number 564) and making up a new “regulatory” pathway that resides entirely outside of all pharmaceutical regulations: NO investigational review board, NO informed consent and NO cGMP compliance apply to things called “EUA countermeasures under Public Health Emergency.”
That’s why when Judge Hughes writes:
Bridges dedicates the bulk of her pleadings to arguing that the currently available COVID-19 vaccines are experimental and dangerous. This claim is false, and it is also irrelevant.
He is correct about “false and irrelevant.” Let’s review how. Medical experiments, aka “clinical investigations” are defined in DFCA as:
Clinical investigation means any experiment in which a drug is administered or dispensed to, or used involving, one or more human subjects. For the purposes of this part, an experiment is any use of a drug except for the use of a marketed drug in the course of medical practice.
However, another US law removes EUAs from the purview of FDCA (and FDA regulations), by creating a special “non-investigational” class:
21 USC 360bbb-3(k): If a product is the subject of an authorization under this section, the use of such product within the scope of the authorization shall not be considered to constitute a clinical investigation for purposes of section 355(i), 360b(j), or 360j(g) of this title or any other provision of this chapter or section 351 of the Public Health Service Act [42 U.S.C. 262].
Therefore, medical experiments are legally not possible for the EUA thingies by their “non-investigational” status. If a product cannot be investigational, there is no process for assembling the regulatory evidence of safety, efficacy, and manufacturing control for purposes of compliance with Section 351(a) of the Public Health Service Act (PHS Act), (42 U.S.C. 262) and cGMP (section 501(a)(2)(B) of the FD&C Act (21 U.S.C. 351(a)(2)( B)) and 21 CFR Parts 210, 211, and 610).
By process of elimination, we arrive at this:
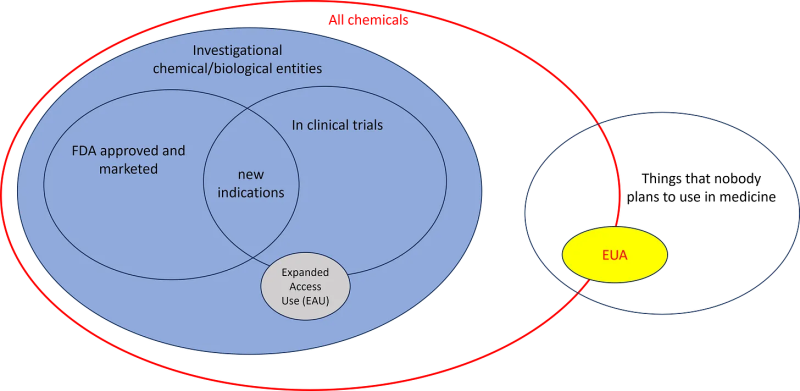

These injections are chemicals and biologics that are not suitable as medicine and nobody plans to use them as such, aka poisons. Please stop calling them experimental, investigational, medical, or pharmaceutical products. They are neither of those things. They are illicit drugs and poisons trafficked by the US Government-Military and their contractors all over the world (video).
Judge Hughes is correct that it is “false and irrelevant” to call Covid injections experimental and dangerous. It is false because EUA is not experimental and can never be. It is irrelevant because safety is irrelevant to EUA, which gets issued based on opinions and not approved based on data. There is no way to collect clinical trial data and collate it such that an assessment of risk benefit (as defined in FDCA) can be made.
Art for today: Still life with a tea kettle, oil on linen 20×20 in.
Republished from the author’s Substack
Join the conversation:


Published under a Creative Commons Attribution 4.0 International License
For reprints, please set the canonical link back to the original Brownstone Institute Article and Author.