1. Introduction
Imagine you are a parent of a child in the age range 12–15 years trying to decide if the benefits of COVID-19 vaccination outweigh the risks. You’ve heard about the link between COVID-19 infection and myocarditis as well as the link between COVID-19 vaccination and myocarditis. You google “myocarditis and COVID-19 infection”. Your search returns the following featured snippet:
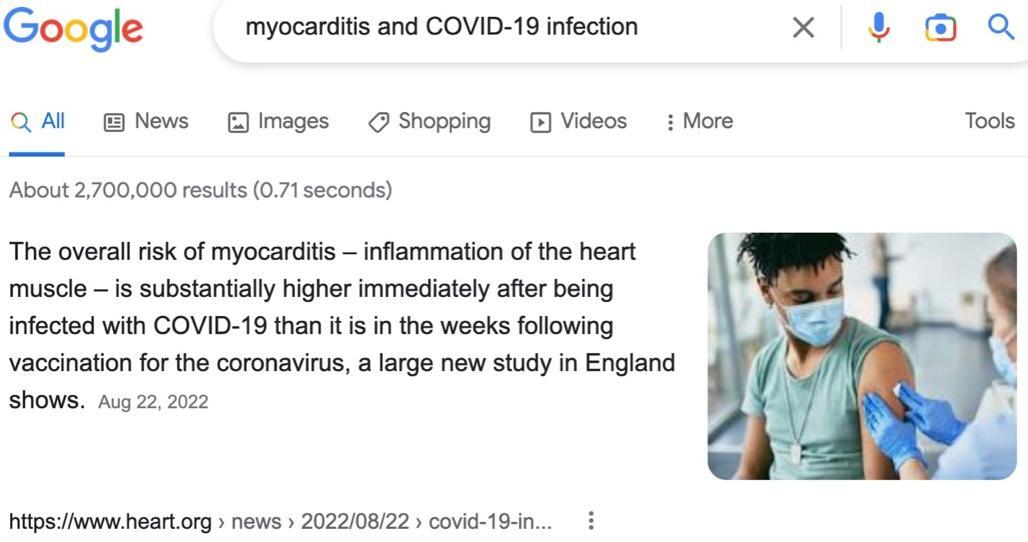

You might conclude that the “best science” suggests your child is at greater risk of developing myocarditis after a COVID-19 infection than after a COVID-19 vaccination. Such a conclusion would be incorrect—two large studies whose findings have been published in prestigious medical journals offer compelling evidence that your child is at a higher risk of myocarditis after COVID-19 vaccination than after a COVID-19 infection; moreover, the “new study in England” providing the information Google has highlighted has serious scientific shortcomings.
The American Medical Association journal Cardiology, 20 April 2022, published a research paper by Karlstad et al. titled “SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents.” In column 2 of eTable 7, we note that within Karlstad et al.’s study population there were 0 cases of myocarditis following SARS-CoV-2 infection for males and females in the age range 12–15. (The study population in the 12–15 age range was “at start of follow-up” 1,238,004, and at the end of the follow-up period 750,253 were unvaccinated.) Moreover, for boys 12–15, eTable 6 reports myocarditis and pericarditis events combined, with 5 events linked to dose 1 of an mRNA vaccine and 6 events, to dose 2.
We will later describe myocarditis data, for children in the age range 13–17, from another large study consistent with that of Karlstad et al.’s for children in age range 12–15. Thus, when a parent searches Google for “myocarditis and COVID-19 infection,” and reads in the top search result that the overall risk of myocarditis is “substantially higher immediately after being infected with COVID-19 than it is in the weeks following vaccination for the coronavirus,” the parent is being misinformed.
Moreover, everyone considering COVD-19 vaccination risks versus those related to infection should be aware that the contrast drawn in the Google-search snippet above between “immediately after being infected” and “in the weeks following vaccination” is extremely misleading. The “new study in England” doesn’t report on myocarditis developing “immediately after being infected”; rather, it reports on myocarditis developing 1–28 days after a positive COVID-19 test, just as it reports on myocarditis developing 1–28 days after a COVID-19 vaccination. In other words, for the study, there is no difference in the temporal association of myocarditis with infection vs. that with vaccination. Hence, the search return is spreading misinformation.
Even worse, the “new study in England” that Google highlights has serious shortcomings.
2. New Study in England: Misleading Assertions
The meaning of COVID-19 infection seems clear—if a person has a nontrivial COVID-19 viral load and may eventually exhibit symptoms of COVID-19 infection, then the person is infected. However, this is not the definition of “infection” used in the “new study in England”. Let’s dig into the details.
The “new study in England” is described in the research paper “Risk of Myocarditis After Sequential Doses of COVID-19 Vaccine and SARS-CoV-2 Infection by Age and Sex,” published 22 August 2022 in the American Heart Association journal Circulation. The paper has 14 coauthors with lead author M. Patone; its “Results” summary begins, “In 42,842,345 people receiving at least 1 dose of [COVID-19] vaccine, 21,242,629 received 3 doses, and 5,934,153 had SARS-CoV-2 infection before or after vaccination.” Patone et al.’s study population consists of 42,842,345 residents of England, ages 13 and up, receiving at least one dose of a COVID-19 vaccine during the study period 1 December 2020 until 15 December 2021. Patone at al. report 5,934,153 SARS-CoV-2 infections occurred in their study population over the period 1 December 2020 until 15 December 2021.
According to a technical article by England’s Office of National Statistics that “presents modelled estimates of the number of people who have had at least one episode of coronavirus (COVID-19),” about 8.3% of the English population had been infected by the beginning of Patone et al.’s study period and about 43.2% had been infected by its end. Thus, roughly, we might expect about 34.9%, (43.2 – 8.3)%, of the study population to have experienced an initial COVID-19 infection during the study period: 0.349 × 42,842,345 ≈ 14,951,978 initial infections, not 5,934,153.
What explains the dramatic undercount of infections in the study population? The following definition of infection adopted by Patone et al., “… SARS-CoV-2 infection, defined as the first SARS-CoV-2–positive test in the study period”. In the context of this study, the preceding definition of infection is not reasonable. Many infections are not associated with (reported) positive COVID-19 tests. For example, the U.S. CDC estimates that the actual number of infections is 4 times the number of reported cases, at least for the period February 2020-September 2021 in the U.S.
How does an undercount of infections affect statistical analysis of the incidence of myocarditis associated with COVID-19 infection? I’ll use data from Patone et al.’s study to illustrate.
As I’ve already noted, the study population consists of 42,842,345 residents of England, ages 13 and up, receiving at least one dose of a COVID-19 vaccine during the study period. Over the course of the study period, 5,934,153 (13.9%) of the study population tested positive for SARS-CoV-2, including 2,958,026 (49.8%) before their first vaccination.
For Patone et al.’s study, a case of myocarditis is one that results in death or in hospital admission for myocarditis—some of these admissions occurred in temporal proximity (1–28 days) to a COVID-19 vaccination, some in temporal proximity (1–28 days) to a positive COVID-19 test, and some, “baseline cases,” did not have either of these temporal associations.
There were 114 myocarditis cases in study-population members while they were unvaccinated that were temporally associated with a positive-COVID-19 test. Based on this raw data, 114 cases resulting from 2,958,026 reported positive tests among study-population members while unvaccinated, we obtain the following incidence of positive-test-associated myocarditis among study-population members while unvaccinated:
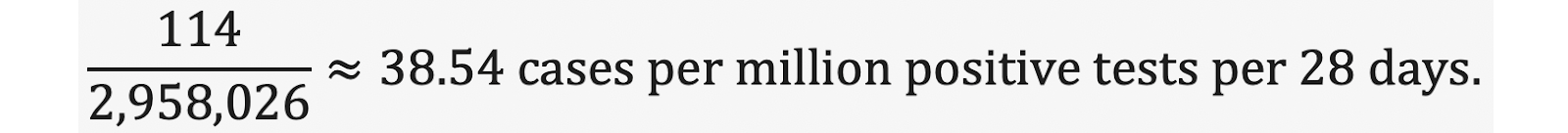
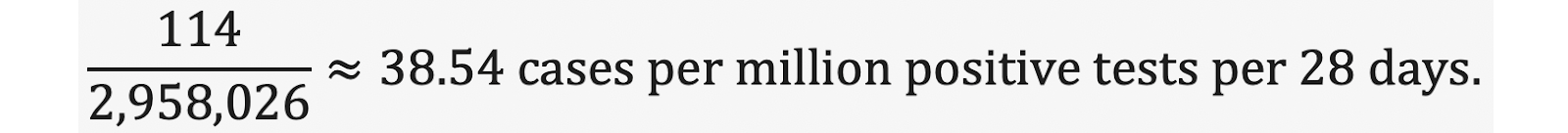
To obtain myocarditis incidence after a COVID-19 infection we must increase the denominator in the preceding quotient so that it reflects the number of SARS-CoV-2 infections that occurred in study-population members while they were unvaccinated. The number of unvaccinated who eventually join the study population starts at 42,842,345 and gradually declines—to approximate the number who become infected before vaccination, we must keep track of the declining number of yet-to-be vaccinated members of the study population as well time-varying rates of infection. This is an interesting math problem, and, fortunately, I’m a mathematician.
A paper that I authored with Spiro Pantazatos describes a computation yielding 4,685,095 as a lower bound on the number of SARS-CoV-2 infections occurring during the study period in members of the study population while they were unvaccinated. Thus, an estimate of the incidence of myocarditis after a COVID-19 infection among study-population members while unvaccinated is


and the preceding is likely to be an overestimate because the method used to compute infections, produces a lower bound on the number of infections based on data from England’s Office of National Statistics (ONS) and National Health Service (NHS).
To understand the implications of using a more realistic count of SARS-CoV-2 infections occurring among members of the study population before they received an initial dose of a COVID-19 vaccine, we’ll assume that the ratio of infections to positive tests, 1.58 ≈ 4,685,095/2,958,026, is similar for the four major demographic groups considered in the study: men under 40, women under 40, men 40 and above, and women 40 and above.
When this factor of 1.58 is taken into account, in, e.g., the incidence rate ratios (IRRs) of Patone et al.’s Table 3, we find that, for men under age 40, the risk of myocarditis after dose 2 of Pfizer’s BNT162b2 (IRR 3.08) is higher than post-infection risk (IRR 2.75, not 4.35) in the unvaccinated, while Table 3 suggests the opposite is true:
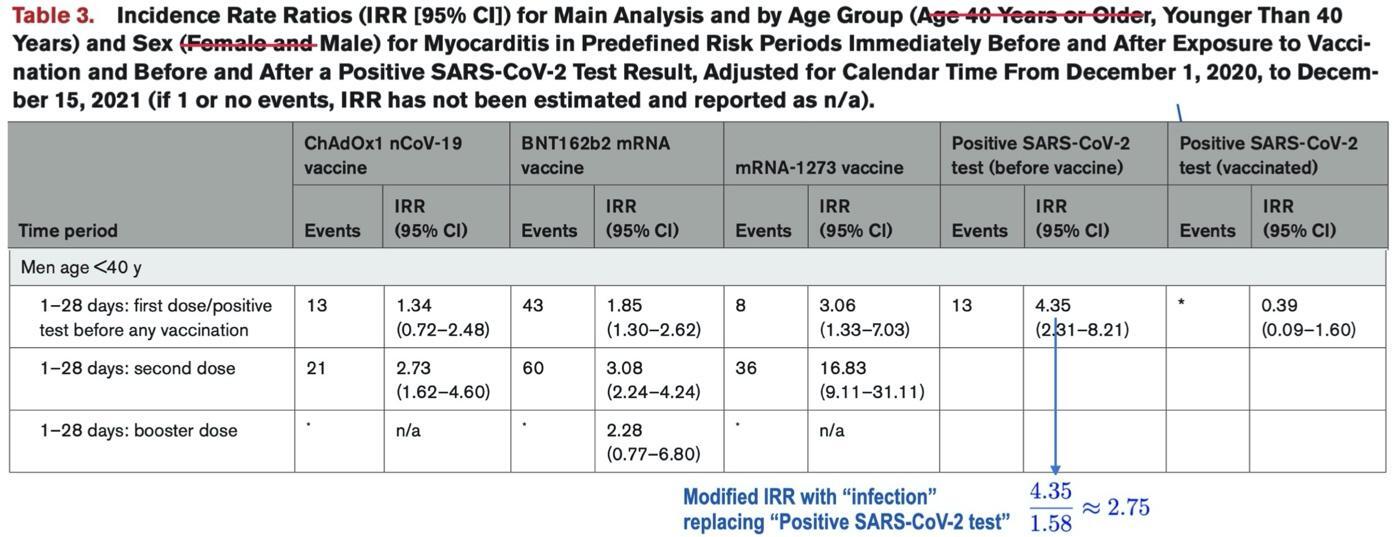

We have modified Table 3 from Patone et al.’s article, eliminating rows corresponding to other demographic groups and adjusting the description of table contents with appropriate strikethroughs.
We remark that others have noticed Patone et al’s study exaggerates the risk of myocarditis after SARS-CoV-2 infection. For instance, Dr. Vinay Prasad raised this issue 28 December 2021 (in commenting on an earlier publication discussing study data from the period 1 December 2020 to 24 August 2021):
While the denominator for vaccines is known with precision, the true number of infections is unknown. Many people don’t seek testing or medical care. So the red bar above [illustrating positive-test-associated excess myocarditis cases] will be shorter if you used a sero-prevalence (aka the correct) denominator.
Patone et al.’s Circulation paper has a number of other serious communication shortcomings, e.g., its failure to appropriately qualify the following statement from the “Discussion” section:
In a population of >42 million vaccinated individuals, we report several new findings that could influence public health policy on COVID-19 vaccination. First, the risk of myocarditis is substantially higher after SARS-CoV-2 infection in unvaccinated individuals than the increase in risk observed after a first dose of ChAdOx1nCoV-19 vaccine, and a first, second, or booster dose of BNT162b2 vaccine.
I’ve already discussed two ways in which Patone et al. should have qualified the preceding statement: “infection” doesn’t mean “infection” and the statement is, with near certainty, false for males and females in the 12–15 age range. Some qualification relating to myocarditis risk in children is offered as a study limitation:
[A]lthough we were able to include 2,230,058 children age 13 to 17 years in this analysis, the number of myocarditis events was small (56 events in all periods and 16 events in the 1 to 28 days after vaccination) in this subpopulation and precluded a separate evaluation of risk.
So, there were 16 myocarditis events associated with vaccination in the 13–17 age group, and, apparently, no cases associated with positive COVID-19 tests, which would be consistent with the findings of Karlstad et al.’s study for the 12–15 age range, mentioned earlier. Note that I have fulfilled my promise to “later describe myocarditis data, for children in the age range 13–17, from another large study consistent with that of Karlstad et al.’s for children in age range 12–15.” Ironically, the other large study providing evidence that children are at higher risk of myocarditis after COVID-19 vaccination than after infection is the “new study in England,” highlighted by Google, to convey that “overall” the risk of myocarditis after infection is “substantially higher” than that after vaccination.
Here’s another extremely important qualification that Patone et al. failed to acknowledge relating to their study’s “new findings that could influence public health policy on COVID-19 vaccination”: Recall that Patone et al.’s study period is 1 December 2020 until 15 December 2021. As Pantazatos and I point out in the section “Additional Limitations of Patone et al.’s Study”, at most 0.18% of the SARS-CoV-2 cases that contributed to the study’s findings were Omicron-variant cases. Thus, the study’s estimates of myocarditis risk following infection do not speak to the risk following Omicron infection, which is recognized to be milder than that of previous variants.
In fact, a recently published study by Lewnard et al. suggests hazard ratios for severe clinical outcomes are reduced across the board for Omicron versus Delta, with hazard reduction “starkest among individuals not previously vaccinated against COVID-19”; e.g., the adjusted hazard ratio for mortality is 0.14 (0.07, 0.28) for the unvaccinated.
Thus, relative to Omicron, we expect that myocarditis incidence rates following infection will be lower even than the appropriately corrected rates based on Patone et al.’s data. By “corrected rates”, I mean those computed using denominators approximating the number of infections rather than the much smaller number of reported positive tests.
Returning to my discussion in the first paragraph of this essay relating to an imagined Google search on “myocarditis and COVID-19 infection,” I suggested that the study cited in Google’s featured snippet does not represent the “best science.” Clear and precise communication with appropriate qualification of statements that might be misused or misinterpreted is certainly a hallmark of good science writing. Patone et al.s’ research paper certainly fails to meet this standard. What about the underlying science of Patone et al.’s study?
3. New Study in England: Flawed Science
The most obvious flaw in the “new study in England” was introduced via a late change in study design, apparently made while Patone et al.’s preprint describing results of their myocarditis study was under review for publication by Circulation. It is my understanding that changing study design after nearly all study data has been collected and analyzed may be a sign of potential author bias.
Moreover, late changes might introduce design flaws that authors have had insufficient time to discover. I describe below a significant flaw in Patone et al.’s study that was introduced after the authors posted a preprint version of their Circulation article on 25 December 2021.
Reading the preprint reveals that, as originally designed, Patone et al.’s study did not include an analysis of the incidence of positive-test-associated myocarditis among the unvaccinated. Rather, positive-test-associated myocarditis events, pre-first-dose and post-first-dose, were combined to compute myocarditis incidence following a positive test independent of vaccination status. Thus, the original study design did not include the flaw discussed below.
COVID-19-related myocarditis risk among the unvaccinated is, of course, unrelated to vaccination. Yet, Patone et al.’s study population consists of vaccinated individuals only. This creates an illogical dependence of Patone et al.’s computation of the incidence of positive-test-associated myocarditis among the unvaccinated on the decision to later vaccinate or not made by a very small number of individuals in England—those individuals, ages 13 and up, hospitalized with positive-test-associated myocarditis during the study period while unvaccinated. Study data shows 114 of those individuals later chose to vaccinate, but we do not know how many chose not to vaccinate. What if none had chosen to vaccinate? Then, the numerator 114 in Patone et al.’s main analysis of incidence of post-positive test myocarditis among the unvaccinated would be 0 and the study would have shown no risk of infection-associated myocarditis among the unvaccinated.
Pantatzatos and I show that Patone et al.’s claimed incidence of positive-test-associated myocarditis among the unvaccinated is valid if and only if unvaccinated persons (age 13 and up) hospitalized during the study period with positive-test-associated myocarditis later chose to vaccinate with the same probability as unvaccinated persons (age 13 and up) who already had a positive SARS-CoV-2 test. We present a plausibility argument that suggests a possible further exaggeration of myocarditis risk post infection by a factor of 1.5. Recall that Patone et al. have already exaggerated post-infection myocarditis risk by dramatically undercounting infections in their study population. A further exaggeration of myocarditis risk post infection by a factor of 1.5 (owing to the study-design flaw discussed above) would, e.g., reduce the IRR estimate computed earlier of myocarditis following COVID-19 infection for men under 40 to 2.75/1.5 ≈ 1.83. which, according to Table 3 from Patone et al.’s Circulation article (relevant portion reproduced in Section 2 above), falls below the IRR for all COVID-19 vaccine doses (including a Pfizer booster) except for a first dose of AstraZeneca ChAdOx1.
I will not offer any speculation as to why Patone et al. made a late change to their study design. Rather, I invite readers to draw their own conclusions based on the comparison provided below of myocarditis-risk data for men under 40 presented in the preprint version versus that presented in the published version in Circulation. First consider the following from the preprint:
Preprint Version, Paragraph Following Table 1: In males aged less than 40 years, we observed an increased risk of myocarditis in the 1–28 days following a first dose of BNT162b2 (IRR 1.66, 95%CI 1.14, 2.41) and mRNA-1273 (IRR 2.34, 95%CI 1.03, 5.34); after a second dose of ChAdOx1 (2.57, 95%CI 1.52, 4.35), BNT162b2 (IRR 3.41, 95% CI 2.44, 4.78) and mRNA-1273 (IRR 16.52, 95%CI 9.10, 30.00); after a third dose of BNT162b2 (IRR 7.60, 95%CI 2.44, 4.78); and following a SARS-CoV-2 positive test (IRR 2.02, 95%CI 1.13, 3.61).
There is no comparable paragraph in the published version—one in which, for men under 40, vaccination-associated myocarditis is compared to positive-test associated myocarditis. However, the portion of Patone et al.’s Table 3 in their Circulation article appearing in Section 2 above, makes the comparison. The paragraph below summarizes the information in Table 3 relating to men under 40:
Published Version, Table 3: In males aged less than 40 years, there was an increased risk of myocarditis in the 1–28 days following a first dose of BNT162b2 (IRR 1.85, 95%CI 1.30, 2.62) and mRNA-1273 (IRR 3.08, 95%CI 1.33, 7.03); after a second dose of ChAdOx1 (2.73, 95%CI 1.62, 4.60), BNT162b2 (IRR 3.08, 95% CI 2.24, 4.24) and mRNA-1273 (IRR 16.83, 95%CI 9.11, 31.11); after a third dose of BNT162b2 (IRR 2.28, 95%CI 0.77, 6.80); and following a SARS-CoV-2 positive test: (IRR 4.35, 95%CI 2.31, 8.21) before vaccination; (IRR 0.39, 95%CI 0.09, 1.60) after vaccination.
Remark: recall that from the discussion of Section 2 above, as well as that of this section, the IRR for infection-associated myocarditis before vaccination is highly likely to be less than 2.75 and possibly less than 1.83.
4. New Study in England: Missing or Miscategorized Myocarditis-Death Data
We now provide a dramatic illustration of the incompatibility of the structure of Patone et al.’s study with an assessment of incidence of positive-test-associated myocarditis for the unvaccinated (in a study-population consisting only of vaccinated persons). We focus on missing or miscategorized data on positive-test-associated myocarditis deaths in Patone et al.’s study population.
One of the myocarditis events tracked in the study is death with “death recorded on the death certificate with the International Classification of Diseases, Tenth Revision code (Table S1) related to myocarditis.”
For death by myocarditis, the event date is the date of death. A person joins the study population only after vaccination, and the person must be alive to vaccinate; so, any person having a record of a positive-COVID-19 test pre-first-dose who joins the population through vaccination will not have a myocarditis death associated with the pre-jab positive test.
Thus, if a study-population member dies from myocarditis, the death will be associated with a vaccination (if within 28 days of the jab), a positive-test (if within 28 days of the test) that occurs after vaccination, or just becomes a baseline myocarditis death. Thus, the only positive-test-associated myocarditis deaths in the study population occur after a breakthrough infection.
Let’s examine myocarditis-death data appearing in Table 2 in Patone et al’s article published in Circulation. The description of table contents suggests the table includes data relating to “SARS-CoV-2 Infection”:
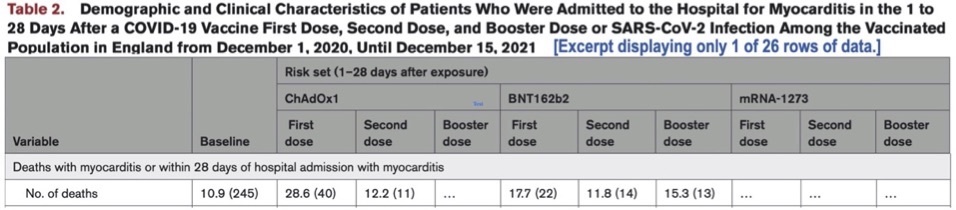

If the preceding table does provide data on “Deaths with myocarditis” associated with “SARS-CoV-2 Infection” (as the table header suggests), where are such deaths recorded? One possibility is that these deaths are in the baseline column (accounting for some of the 245 baseline deaths), but that would be a miscategorization, equivalently, a misrepresentation of fact.
I suspect the data is simply omitted. Why? If infection-associated myocarditis-death data were included, then it would be obvious that Patone et al.’s separate analysis of positive-test associated myocarditis events pre-first-dose vs. post-first-dose is incompatible with the principal inclusion criterion for their study population—receiving one or more doses of a COVID-19 vaccine during the study period.
Consider the following excerpt from Supplementary Table 2 of the preprint version of Patone et al.’s Circulation article.
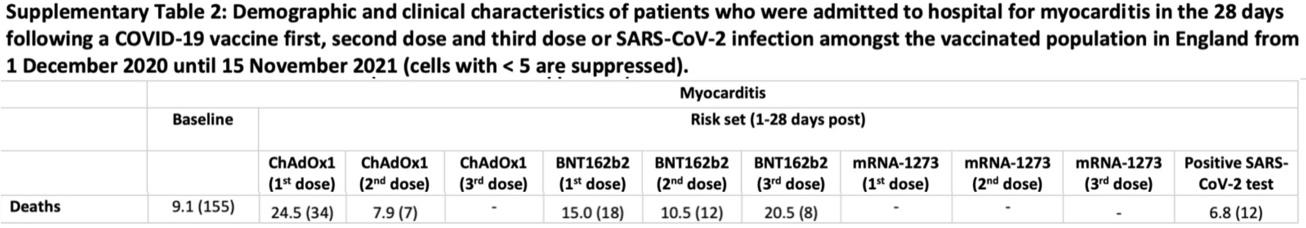

We see that there were 12 positive-test-associated deaths in the study population during the period 1 December 2020–15 November 2021, so that there are necessarily ≥ 12 positive-test-associated deaths in the study population during the full study-period 1 December 2020–15 December 2021 of Patone et al.’s published article. As discussed above, the structure of Patone et al.’s study is such that all positive-test associated myocarditis deaths must occur post vaccination.
Thus, given the way Patone et al. chose to analyze positive-test-associated myocarditis for their published study and assuming that positive-test-associated myocarditis deaths are not inappropriately included in baseline deaths, a table providing a complete report of the death-by-myocarditis study outcome would include a number-of-deaths row having the form illustrated below:
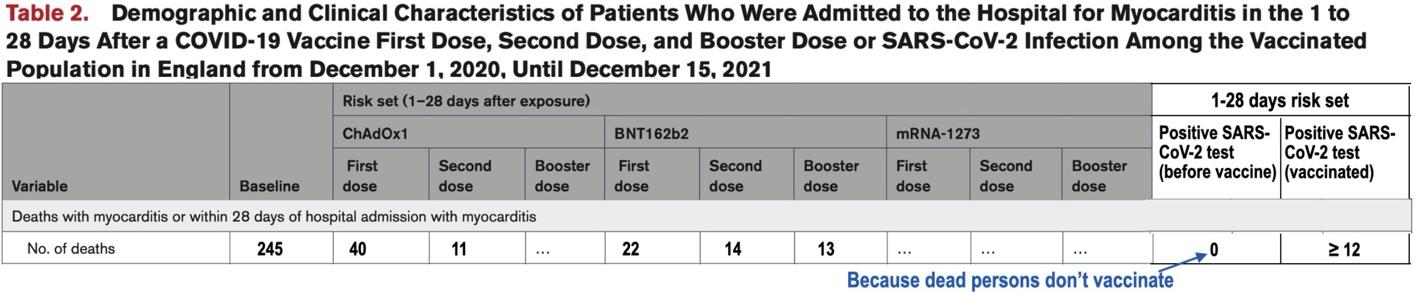

The preceding table illustrates why a complete and accurate report of the death-by-myocarditis study outcome was not included in Patone et al.’s published Circulation article—such a report clearly shows how incompatible the structure of Patone et al.’s study is with an attempt to analyze the incidence of positive-test-associated myocarditis for the unvaccinated (in a study-population consisting only of vaccinated persons). Why did Patone et al. make the decision to modify their study design to include such an analysis, and apparently while their Circulation submission was under review for publication?
5. Conclusion
Let’s return to Google’s highlighted response to the search request “myocarditis and COVID-19 infection”:
Featured-snippet response: The overall risk of myocarditis – inflammation of the heart muscle – is substantially higher immediately after being infected with COVID-19 than it is in the weeks following vaccination for the coronavirus, a large new study in England shows.
Because the “study in England” (by Patone et al.) uses a misleading definition of “infection” (see Section 2 above), has a serious design flaw introduced after nearly all study data had been collected and analyzed (see Section 3 above), and nearly all infections occurring in the study population were not Omicron infections (see Section 2 above), it’s possible that the assertion of the snippet above is false in complete generality—post vaccination risk may be higher than post Omicron-infection risk for all age groups, males and females. In this essay, I have established the snippet assertion is, with near certainty, false for children in the age range 12–15 and highly likely to be false for, say, a male under 40 contemplating receiving a second dose of Pfizer’s BNT162b2.
Why did Patone et al. use a misleading definition of “infection”? Why did they change their study design after nearly all study data had been collected and analyzed? Why did they fail to emphasize that their finding of the snippet doesn’t apply to children in the age range 13–17 years? Why did they fail to acknowledge that their finding of the snippet above may no longer be valid relative to Omicron infection?
Here’s an even more important question: Why is the medical establishment so poorly informing the public about risks of myocarditis post vaccination versus post infection?
I’ll conclude with some general observations about comparing risks of COVID-19 vaccination to similar risks of COVID-19 infection. Vaccination with an mRNA COVID-19 vaccine includes risks associated with two doses, and likely booster doses. Thus, e.g., myocarditis risk following infection should be compared to the combined risk of at least doses 1 and 2 of an mRNA vaccine.
A comparison of a risk associated with COVID-19 infection to the same risk associated with COVID-19 vaccination should not be restricted to only the 28 days following infection or vaccination. If vaccination prevented infection and repetition of vaccination weren’t required, then limiting assessment of a risk linked to infection versus the same risk related to vaccination to a short window during which adverse outcomes typically occur seems reasonable.
However, in the long run, COVID-19 vaccination provides little or no protection from infection. (E.g., see Table 4 of the UK Health Security Agency’s COVID-19 vaccine surveillance report of 3 November 2022.) Thus, an analysis of risks versus benefits of vaccination must assess to what extent will vaccination reduce the number of infections a vaccinated person will experience and to what extent, if any, will vaccination reduce the incidence and/or severity of adverse outcomes associated with infections.
Join the conversation:


Published under a Creative Commons Attribution 4.0 International License
For reprints, please set the canonical link back to the original Brownstone Institute Article and Author.









