This week, the US Food and Drug Administration (FDA) authorised the use of a single booster of Pfizer’s covid-19 vaccine in children aged 5-11, at least five months after completion of a primary series of vaccinations. The CDC’s advisory committee is expected to rubber stamp the decision today.
According to the FDA, the evidence underlying its decision came from a subset of 67 kids who were part of an ongoing trial and were boosted 7-9 months after their primary series. They showed higher antibody levels one month after the booster compared to before the booster.
The decision by the FDA is audacious for several reasons.
Lack of data
Just when the FDA should be demanding better data, the agency lowers its standards.
No rigorous studies in this age group have shown that a third dose can reduce important outcomes such as hospitalisations and deaths – the randomised clinical trials have not been done, despite Pfizer earning billions in revenue.
Instead, the decision was based on the presence of “neutralising antibodies” because they are easy to measure and study. Not only do antibody levels fade quickly, but they also do not necessarily correlate with protection.
The FDA’s own website says that “antibody tests should not be used to evaluate a person’s level of immunity or protection from covid-19.” And yet, this is what the agency has done.
This follows previous data from New York during the omicron surge showing Pfizer’s vaccine effectiveness in 5-11-yr olds plummeted from 68% in mid-Dec 2021, down to only 12% by Jan 2022, well below the FDA’s original threshold of 50%.
The myopic focus on antibodies by drug regulators and health authorities, has been at the expense of considering other important aspects of the immune system such as CD4+ T-cells and natural killer responses, which play a crucial role in preventing infection and are thought to be more durable than antibodies. Unfortunately, these data have been largely ignored by authorities.
Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research acknowledged that data increasingly shows that protection after two-doses wanes over time, and that a third shot could help boost protection for children in the 5 to 11 age group because the “benefits outweigh the risks.”
However, in terms of safety, the FDA has only assessed approximately 400 children who received a booster dose and any serious, rare harms would not be detected in such a small sample size.
Ignoring natural immunity
By authorising a third dose for all 5-11 yr olds – most of whom already have natural immunity – is unlikely to provide further benefit and may expose them to unnecessary harm.
The US CDC reported that about 75% of children and adolescents have serologic evidence (antibodies) from a previous infection, and therefore, have already developed robust and durable protection against covid-19.
The UK government estimated that over 85% of children aged 5–11 years had contracted covid-19 by January 2022 and that their acquired natural immunity would provide protection against severe disease or future re-infection.
Marty Makary, professor at Johns Hopkins School of Medicine wrote in The Wall Street Journal that natural immunity is likely to be very robust in children given their stronger immune systems. He said that if a child already had had covid-19, “there’d be no scientific basis for vaccination.”
He also observed that no cases of covid-19 were documented in either the vaccinated group or the placebo group in children that had been previously infected with SARS-CoV-2 during Pfizer’s trial, reinforcing the benefit of natural immunity.
Advisory panel
The Vaccines and Related Biological Products Advisory Committee (VRBPAC) not only provides insight and expertise to the agency, but it lends credibility and trustworthiness to the FDA decision-making process.
However, the FDA did not convene its advisory panel this week, saying that it had already discussed boosters at a prior meeting and that further discussion would not be of any benefit.
Some members have raised concerns that the drug regulator has repeatedly moved ahead with decisions on booster doses without holding open public discussions and say the agency is relying less and less on its independent experts for advice before approving drugs.
In a recent analysis, researchers showed that only 6% of drugs approved by the FDA were reviewed by advisory panels in 2021, down from 55% in 2010 (see figure).
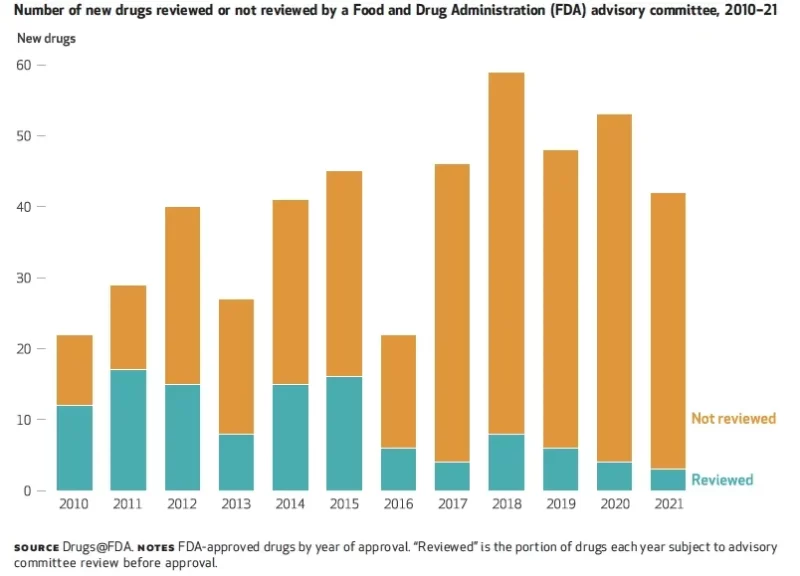

If public confidence mirrors these figures, regulatory agencies have a long way to go in regaining trust in their decision-making processes.
Uptake?
Despite the continued expansion of boosters, enthusiasm for the vaccines seem to be waning. The covid-19 vaccines are not as effective at preventing infection and transmission as originally hoped, and with such a small risk of serious disease in children, parents are becoming less convinced.
A recent US survey found almost a third of parents of children under 5 years said they would “definitely not” get their child vaccinated in the first place, another 11% stated they will only do so if required (mandated), and 38% planned to wait in order to see how the vaccine worked for others.
Pfizer has yet to submit its application to the FDA on a three-dose vaccine for kids under 5, but it is expected to do so, in the coming weeks.
Originally posted on the author’s site
Join the conversation:


Published under a Creative Commons Attribution 4.0 International License
For reprints, please set the canonical link back to the original Brownstone Institute Article and Author.









