Early in the pandemic, President Donald Trump and White House senior official Peter Navarro arranged the donation of 63 million doses of hydroxychloroquine (HCQ) to America’s strategic drug stockpile to combat Covid-19. The government began securing HCQ in March 2020, after Trump, on the advice of his medical and scientific advisors, lauded HCQ as “very encouraging,” “very powerful,” and a “game-changer.” While HCQ (and its structurally similar analogue chloroquine) was not FDA-indicated for Covid-19, it was well-known to have specific off-label pharmacological functionality for preventing viral particle entry into cells, chemical derivatives of which have been utilized for antiviral use as far back as 1934.
Following Trump’s proposal, HCQ suddenly came under an unwarranted full-scale attack from federal officials, the press, so-called “fact-checkers,” and university professors. Many of the attacks contained outright falsehoods about HCQ’s pharmacology and safety or Trump’s endeavor to make HCQ available to eligible patients.
The FDA initially issued an emergency use authorization (EUA) for HCQ in March 2020, but withdrew authorization on June 15th 2020, stating the drug is “unlikely to be effective in treating COVID-19 for the [EUA] authorized uses.” Around the same time, the FDA also wrote a methodologically questionable report criticizing HCQ’s safety. The FDA’s narrative was based on preliminary and time-compartmentalized findings, and not a reflection of historical safety or based on the appropriate clinical use of HCQ dosing, prescribing, timing, and duration. The FDA then seemed to label its findings as conclusive, figuratively slamming the door shut on the consideration of new findings.
FDA Hydroxychloroquine Safety Assessment Based on: Known Overdoses, and Clinically Unsupervised Uses
According to its uncharacteristically brief 15-page safety review memorandum of HCQ published on May 19, 2020, the FDA considered data from the National Poison Data System (NPDS) which means it seems to have included the use of non-pharmaceutical-grade and/or self-administered HCQ and/or overdose data in its clinical evaluation.
Obviously, overdose or any non-medically supervised, self-diagnosis, and/or self-dosing of any prescription drug has a higher potential to result in adverse events, especially because the antiviral dose/duration of HCQ and/or chloroquine were not immediately obvious to the lay public. Of note, “if it bleeds it leads”-type sensationalized news reporting caused anxious and desperate Americans with incomplete or incorrect transmission and mortality information to go so far as to self-administer “fish tank” chloroquine-containing cleaning products of containing other chemicals to treat themselves from possible Covid-19 exposure. This led to severe illness due to the ingestion of toxic ingestions or overdoses, sometimes up to the point of death.
Heavy Reliance on the FDA’s Adverse Event Reporting System Database
In its report, the FDA also referenced its Adverse Event Reporting System (FAERS) which according to Table 6 only contained around 256 applicable adverse event reports over a five-month period in the entire world when administered for Covid-19. Of note, most of the reports cited by the FDA included doses which were either unknown and/or were anywhere from 2x to 6x the recommended maximum maintenance dose of HCQ for any clinical indication per FDA and manufacturer’s dosing recommendations. On top of that, it appears that the FDA gave no consideration to HCQ base/salt formulations or critical weight-based dosing of HCQ (on a mg/kg basis) as it did not appear to investigate or report patient weights. It is unknown if any organ function assessments were considered as part of their safety collection or evaluation – an important consideration since HCQ is hepatically metabolized and renally excreted, with dosage adjustments potentially needed for out-of-range liver or kidney function.
These and other important mitigating factors would have been needed for a suitable evaluation of HCQ safety by the FDA.
FDA Seemed to Include Well-Known Drug-Drug Interactions and Unknown Quality Likely from Rogue Internet Pharmacies:
Well- established drug-drug interactions, some established as far back as the 1980s, occurred in individuals who obtained HCQ by circumventing normal patient/prescribing channels. In addition to the incorrect/excessive dose/durations used, the FDA’s report details how HCQ appeared to have been dispensed from rogue overseas pharmacies which have a history of producing consumer drugs with poor quality and toxic contaminants.
In its report, the FDA showed that the majority of all adverse event cases (69% of the 331 total HCQ safety reports) involved males with a median age in the early 60s, yet the FDA used those negative findings to recommend against the use of HCQ in every age group.
On top of that, Page 7 of the report showed most of the 109 serious HCQ cardiac adverse event cases cited by the FDA also directly contraindicated well-established FDA and/or manufacturer dosing guidelines for HCQ.
Specifically:
- 92 of the 109 serious cardiac cases (84%) reported concomitant use of at least one other medication that prolongs the QT interval.
- 75 (69%) of cardiac cases specifically reported concomitant use of azithromycin.
- 22/25 [88%] of the fatal cases reported use of a concomitant QT-prolonging medication.
In other words, the FDA included well-known, clinically unsound, long-established, drug-drug interactions, contraindicated uses, and/or prescribing errors in denigrating HCQ’s safety.
It is fair to say that 84% of cardiac adverse events (and possibly the majority of other reported HCQ adverse events listed in the FDA’s memorandum) could have been prevented by clinical patient education, appropriate clinical supervision, and the appropriate pharmacist dispensing/counseling of a medication of known quality from a US pharmacy, including cardiac assessments and the appropriate checks for drug-drug interactions. Even more adverse events could have likely been prevented with a brief cardiac history evaluation and/or an electrocardiogram. In fact, Lopinavir/ritonavir (Paxlovid), used to treat Covid, has an established QT prolongation effect like HCQ, but since warnings about drug-drug interactions are well-publicized and Paxlovid is dispensed under physician/pharmacist supervision, reported cardiac adverse events are uncommon, avoiding cardiac and other safety concerns.
No one drug is ever appropriate for every single person, and not everyone is eligible to be dosed with HCQ for underlying reasons including organ function, cardiac conditions, and/or the risk for established adverse events. However, drug-drug interactions and adverse events can be mitigated by dose adjustment and/or temporarily pausing other pharmacotherapies for the limited duration of time that HCQ is needed to prevent or treat early Covid-19 post-exposure. Separately from that, other FDA admonishments regarding safety described in its report appear to instead describe those related to long-term dosing not needed for Covid-19 early- or pre-exposure indications or temporary and widely touted “stop the spread” initiatives.
Hydroxychloroquine Is Objectively Safe When Used Appropriately:
Following the unauthorized pandemic surge of HCQ use, a clearer message from America’s FDA to the American public about the safe uses of HCQ should have taken place but didn’t. Instead, the FDA kept silent, did not specifically warn consumers about circumventing America’s medical and pharmacy system, and let Americans make (sometimes fatal) HCQ-related usage errors. The FDA then released a surveillance memorandum essentially pronouncing HCQ as “unsafe” for Covid-19. The FDA made that safety declaration despite the CDC promoting the use of HCQ back in 2019 as safe and “a relatively well tolerated medicine.”
In fact, HCQ is considered so safe for non-Covid-19 indications that the CDC states that “HCQ can be prescribed to adults and children of all ages. It can also be safely taken by pregnant women and nursing mothers.” The CDC was referencing the long-term use of HCQ for chronic disease treatments.
If the CDC considers it safe for long-term treatment, it is only logical to assume that it would certainly be safe for short-term use against quickly spreading viral infections like Covid-19.
Hundreds of other studies (listed in the bibliography of this paper) have been shown to only rarely report safety concerns during limited duration of HCQ administered for Covid-19. Of those, almost all were minor, and of those, all appeared to resolve upon drug discontinuation. The specific reports of HCQ safety are detailed in the summaries following each citation in the bibliography.
FDA Duplicity: FAERS Is OK to Vilify Hydroxychloroquine Safety, but NOT OK to Denigrate mRNA Injection Safety:
The FDA’s heavy reliance on AERS reports in denigrating HCQ in its report was not only biased – it was ironic.
In the past, the FDA and the NIH have repeatedly scolded the use of FAERS as having “not been verified” and “not establish[ing] causation” and that “correlation is not causation” as an excuse to seemingly selectively ignore correlation, and how FAERS “Rates of occurrence cannot be established with [FAERS] reports” and how FAERS findings have “no definitive proof of the causal relationship between exposure to the product and the reported event.”
Therefore, by the FDA’s own account, any of the supposedly valid 256 HCQ FAERS cases cited in its reports could have been:
1) Exaggerated,
2) Not allowed to be used to calculate or imply an incidence rate, (which the press did anyway),
3) Attributable to causes other than HCQ and
4) *If* used to calculate rate, the potential calculated incidence rate of 291 cases worldwide would represent an exceptionally small relative adverse event incidence, but we don’t know that because the FDA did not provide or estimate the 291 cases versus the total number of patients dosed. In other words, the 291 figure is the numerator, but what is the denominator?
The FDA based its safety decision on a worldwide solicitation of a total of 331 reports from all sources (a huge fraction of which were obvious clinically inappropriate uses and/or overdoses and/or in people over the age of 60), over an approximate five-month period, including 256 worldwide reports in FAERS, including: 25 total reports in the entirety of published medical literature, 20 reports form the NPDS, and 11 “other” reports of nebulous origin. One-hundred and nine total HCQ/chloroquine were adjudicated as “serious cardiac related” and an additional 113 were “serious non-cardiac.”
No indication was given that non-fatal adverse events didn’t fully resolve following short-term HCQ use discontinuation or the full course of treatment to prevent or treat Covid-19.
As is clear, the adverse events that occurred had mitigating factors. HCQ is considered to be a safe drug with relatively few adverse event reports. In fact, a comprehensive search for the total number of adverse event reports for HCQ/chloroquine over the past 55 years of worldwide use (including very minor adverse events and those adverse events originating from known drug-drug interactions) in the FDA AERS database showed a grand total of 32,011 cases according to the most recent database update in 2024. The clinical use of chemical precursors of HCQ date back nearly 100 years, but safety and adverse event collection databases only date back to around 1969.
Relative Safety of Hydroxychloroquine’s 32,011 vs >1,000,000 mRNA FDA Reports:
While 32,011 adverse event reports is not inconsequential, compare that to the over 1 million adverse event reports submitted to the FDA’s Vaccine Adverse Event Reporting System (VAERS) for mRNA Covid-19 shots, total, just since 2021 (i.e. ~3.5 years) – and not in the whole world – but in the United States alone, with tens of thousands of those million known to be serious, permanent, and/or deadly.
In fact, the number of worldwide deaths reported from mRNA injections (over 37,500) during its three years on the market exceeds the total number of reported adverse events that occurred during the entire 55-year history of HCQ use. Of note, an abundance of HCQ adverse events associated with the short-term use of HCQ were minor, and included things like nausea, decreased appetite, and fatigue, which are adverse events associated with many different drugs.
Despite that, around the same time that the deluge of mRNA adverse events were reported, America’s self-proclaimed “expert fact-checkers” repeatedly used their megaphones to scold Americans that HCQ was “unsafe.” Major medical research centers and fact-checkers told Americans that numerous mRNA adverse event reports and unexplained sudden deaths and clinical reports of cancers were “not causation” and on top of that, Covid mRNA shots are additionally “Proven Safe” and “Not Dangerous” and “Do NOT Need To Be Withdrawn From The Market” [capitalization theirs].
One does not need to be an expert in drug safety epidemiology to distinguish the incongruity between the continued EUA followed by full approval for novel mRNA Covid injections with hundreds of thousands of adverse events, compared to rapid HCQ EUA withdrawal following 331 worldwide HCQ adverse event reports, an abundance of seemingly related to improper sourcing/use/dosing/supervision.
Major Journal Articles Declaring Hydroxychloroquine as Unsafe for Covid-19:
Prior to HCQ’s EUA removal, a seemingly highly coordinated and unquestionably harmonized message came out against HCQ from American’s press, making it appear that Trump’s HCQ recommendation was not only “unsafe” but that it also “didn’t work” for Covid-19. Harvard, Stanford, and Scripps Institute scientists (respectively) warned Americans via a Washington Post article that Trump’s efforts to employ HCQ were “desperate” and “If there was ever hope for [HCQ], this is the death of it” and “It’s one thing not to have benefit, but [HCQ] shows distinct harm.”
The scientists quoted above were referencing Lancet and New England Journal of Medicine articles that were widely referenced as a means to criticize Trump’s proposal for implementation of HCQ for Covid-19. Both publications were later retracted by journal editors due to being fraudulent.
They were retracted by the journals when its authors “refused to give [auditors] access to all the data they asked for” following publication when results were questioned by outside scientists who questioned why HCQ, with such a historical safety record suddenly appeared so unsafe for Covid-19 patients. Questions led to an investigation which eventually revealed that none of the publication’s authors nor journal “peer-reviewers” had likely ever seen the 96,032 patient data in the first place, because it never existed. The critical question is: why did those so-called “peer-reviewers” permit publication of highly incongruent safety findings for HCQ before thoroughly confirming those findings?
Following the redaction, Lancet’s editor, Richard Horton, stated he was appalled with the authors, calling the HCQ-lambasting study “a shocking example of research misconduct in the middle of a global health emergency.” Lancet’s editor did “…apologise to the editors and to readers of the Lancet for the difficulties that this has caused.”
The same press that had no reservations about hysterically labeling the Trump administration as wrong for attempting to advance HCQ and coordinating messaging against him was almost completely silent and of course did not coordinate or harmonize any correction admitting that they had not verified highly questionable data even though people’s lives were at stake.
It is known today that the press and medical journals were not just wrong but outrageously wrong, about both their declaration and the methodology they employed to arrive at their conclusions despite supposedly delivering “real truth,” being “fact-checked” and delivering “real facts,” and/or claiming to be “peer-reviewed research.”
Consequences for Publishing False Hydroxychloroquine Data?
The now retracted articles had been primarily authored by Mandeep Mehra MD, a Harvard Medical School professor who also serves as the director of the Brigham and Women’s Hospital Heart and Vascular Center. On a side note, Dr. Mehra retains both positions/titles to this very day, in what has become an all too familiar pattern of Harvard and other prominent university officials maintaining their prestigious and lucrative positions despite publication fraud and/or incompetence.
The second author on both papers was Sapan Desai MD, who had claimed to have the world’s largest and most sophisticated patient databases, under his now defunct Illinois-based company, Surgisphere. As it turns out, his database which had reported harmful effects tied to HCQ among patients with Covid-19 never existed, and the “peer-reviewers” at the supposedly “top tier” Lancet, and New England Journal of Medicine whose job it was to critically review the data never actually verified any of the highly questionable epidemiology findings contrasting HCQ’s legendary safety record in autoimmune disorders and malaria, as detailed in an abundance of publications plus FDA’s AERS database.
The third author of the paper, Professor Frank Ruschitzka MD, (like Harvard’s Mehra) still holds his Chairmanship of the University Heart Center and the Department of Cardiology at the University Hospital in Zürich, Switzerland.
The fourth author on the paper, Amit Patel MD is related to Sapan Desai through marriage. He is the only author who has been directly “punished” for publishing fraudulent data, having “mutually agreed” to having had his unpaid, adjunct faculty position at the University of Utah, terminated.
Further investigations into Sapan Desai by others found multiple, egregious incidences of medical fraud along with multiple incidence of clinical malpractice and negligence preceding his Lancet publication — something that journal peer reviews should have been tuned in to and considered while reviewing his data for publication.
Although Dr. Desai was allowed to voluntarily surrender his medical licenses in Ohio and Illinois, it appeared to be related to patient care-related matters. An internet search did not show any pending litigation against Dr. Desai for fraudulently publishing medical data. It is unclear if Dr. Desai or any other authors are facing criminal charges for falsifying negative clinical findings about HCQ.
It is unknown what if any ramifications occurred to either journals’ “peer-reviewers” as a result of allowing these fraudulent safety-incongruent HCQ data to be published.
Blind Regurgitation of Faulty Journal Publications by the Lay Press:
Politicians and “trusted journalists” with zero education or training in science – let alone no background in pharmacology – let alone no background in the intricacies of investigational medicine, epidemiology, or the clinical assessment of drug safety – exuberantly leapt to criticize Trump’s HCQ proposal, based on both unverified or fraudulently written journal publications.
Here are just some of the many quotes:
- The New York Times stated that Trump’s HCQ efforts were “Likely for nothing” and gushed about how “Medical experts across the country… applauded the FDA’s withdrawal of the [emergency use] waiver” in referring to HCQ.
- Another Washington Post article stated that HCQ use for Covid-19 “makes no sense” and “no medical evidence supports Trump’s hydroxychloroquine obsession” in a piece titled: “…People who take his [Trump’s] advice may die” in referring to HCQ.
- ABC News stated that Trump’s recommended use of HCQ for Covid-19 “directly contradicted guidance from the nation’s top public health agencies and officials.”
- The medical and pharmacology heavyweights at Salon.com opined that HCQ was “Revealed to be useless for treating COVID-19” stating that Trump’s “[HCQ] stockpile epitomizes presidential incompetence.”
- Arizona Democratic Representative Raul Grijalva adjudicated HCQ as “useless” stating that Trump used “lies and falsehoods” promoting HCQ, seething that “Trump creates a crisis everywhere he goes and consistently puts his own desire to be right above the health needs of everyday Americans.”
- The reliably gormless Arizona Republic journalist E.J. Montini, who for years, can barely write an article not denigrating Trump, also opined on drug safety, calling Trump’s followers “hydroxychloroquine kooks” adding his smarmy epithet for Trump’s medical experts (ostensibly including Yours Truly) as a “coterie or bootlicking minions” [SIC]. Montini trusted regurgitated propaganda from other lay reporters regarding the Lancet and the New England Journal of Medicine as nothing short of biblical wisdom, beyond any deliberation, discussion, or critical analysis.
- Former independent presidential candidate Robert F Kennedy, Jr (RFK) was also called out for his promotion of HCQ. RFK was chastised as recently as a few days ago, on August 23, 2024 by “fact-checkers” at the putatively venerable University of Pennsylvania in a piece titled: “RFK Jr.’s COVID-19 Deceptions” which stated “As we’ve written, clinical trials of both ivermectin and hydroxychloroquine have shown no evidence of effectiveness against COVID-19”…despite the analysis of hundreds of HCQ studies involving hundreds of thousands of patients detailing the exact opposite and with high statistical probability. How peculiar that not even one scientist or physician from University of Pennsylvania’s Perelman School of Medicine (which incidentally has dedicated epidemiology and biostatistics faculty) were seemingly consulted, referenced, or quoted anywhere within its “fact-check” declarations, especially since the authors do not appear to have formal statistical/ epidemiological/medical/pharmacology/pharmacy training themselves.
Timeline of the Distortion Hydroxychloroquine Safety, Almost Immediately Following Trump’s Proposal:
In case anyone has forgotten about the actual, carefully worded declarations that President Trump made, delicately suggesting that data had shown that HCQ could be useful for Covid-19, here are his actual statements made during his press conference:
Trump directly stated during a press conference on March 20, 2020 that he proposed to use HCQ for early treatment “at the beginning” of Covid-19 infections at 0:22 in the video above. Trump was right to suggest that because today, there is a great deal of evidence that early (or even prophylactic) treatment with HCQ is very effective for Covid-19.
During the same March 2020 press conference and standing alongside Trump, Fauci very accurately stated that “[HCQ] toxicities are rare, and in many respects, reversible” at 1:50.
Following Trump’s proposal and subsequent stockpile of product, HCQ experienced a stunning, seemingly coordinated, fall from favor.
First, Fauci changed his mind regarding his March 2020 statement following the publication in the New England of Medicine on May 1, 2020 (later retracted), the FDA’s problematic methodologies in its review on May 19, 2020 (discussed above), and the Lancet’s publication on May 22nd, 2020 (later retracted).
Despite historical evidence of HCQ being both safe and effective, physicians, politicians, and organizations, taking their lead from incorrect narratives from Fauci, the press, and medical publications, rushed to parrot outrageously incorrect anti-HCQ on top of highly emotional anti-Trump narratives.
The following are just a few of the dozens upon dozens:
- Four months after stating the exact opposite during the above press conference video, on July 29, 2020, Dr. Anthony Fauci told CNBC that there was “no evidence it was effective.” Fauci also suddenly shifted, adding that HCQ for Covid-19 “didn’t make scientific sense.” Seemingly not critically evaluating or looking beyond (falsified and now retracted) New England Journal and Lancet conclusions.
- Josh Cohen, a Forbes.com PhD senior healthcare columnist (with a background in economics) later headlined an absurdly biased op-ed based on a study out of France stating that Trump’s HCQ proposal could be “Linked To 17,000 Deaths” …except Cohen left out the “could be” part. Forbes’s Tufts- Harvard- and University of Pennsylvania- trained “healthcare analyst” (who also opines on climate change and the Inflation Reduction Act) fully neglected to mention that such a number was fully theorized, conjectural, and that estimation was an extrapolation of a late-stage “compassionate use” scenario where HCQ could have, hypothetically caused an 11% increase in mortality. He also chastised the “unproven, experimental nature of hydroxychloroquine” even though non-experimental and proven positive clinical findings existed prior to his piece being published. Of course, one would never know that by Forbes’ appallingly misleading article title which instead of being an “analysis” seemingly closely paralleling the writings of non-”healthcare analysts” at The Hill and Politico. The original publication upon which Cohen’s (and The Hill’s and Politico’s) article was based, was (in what has become a familiar pattern) retracted at the request of the publishing journal’s editor-in-chief following an investigation of journal subscribers which uncovered major deficiencies in the study’s dataset (and other failings). Despite that, the above (in addition to other) news articles remain live and online, accumulating hits from a public that believes them to be factual and current, still used as a talking point against Trump. Will redactions of these badly outdated op-eds ever take place?
- Physician and outspoken medical pundit Dr. Vinay Prasad, a University of California at San Francisco oncologist stated via a MedpageToday.com op-ed: “And, yes, let’s put it to bed: hydroxychloroquine doesn’t work for COVID,” while lamenting within that same MedPageToday article that anyone was even permitted a voice to be given to an opposing side in the Covid-19 debate. Like others, Prasad had slammed the door shut following preliminary findings. Prasad seemed to perform rote parroting of the opinions of others in place of a comprehensive or original review of the data as one would expect from an outspoken physician. In the same article, he also compared giving a voice for alternate Covid-19 treatments like HCQ to giving a voice to “flat-earthers.” Dr. Prasad also stated via a Washington Post op-ed that “Trump’s medical judgment is wrong. The example he’s setting is worse.” He also stated via Twitter/X that “The prior administration loved hydroxychloroquine and ivermectin and other foolish unproven drugs.”
- Yale University’s Department of Epidemiology’s Dean, Dr. Stan Vermund quoted a line, admonishing against the use of HCQ for Covid-19 on July 29, 2020 stating “[HCQ] showed no benefit for decreasing the likelihood of death or speeding recovery.” His widely-cited letter remains online, prominently showing up in internet search results to this very day. Dr. Vermund was chastising HCQ research published by Dr. Harvey Risch, a distinguished epidemiologist and Yale colleague, while failing to directly link Dr. Risch’s publication and also not specifically listing any fault with Dr. Risch’s research methodology. At the time, well-conducted studies contradicted Dr. Vermund’s quote and sentiment against HCQ, with dozens upon dozens more studies since released, but no updated or revised letter from Dr. Vermund addressing those favorable data.
- The WHO stated that HCQ “has no meaningful effect on deaths or hospitalizations” and “made a strong recommendation against the use of hydroxychloroquine” and that they “…do not consider this drug worthwhile.”
- The WHO later stated in March of 2021 that “more than 80 trials planning to enroll at least 100,000 participants to further research hydroxychloroquine are unlikely to uncover any benefits and should be canceled.” It appears that the WHO’s mind was made up, and that even potential new data examining different doses/endpoints/HCQ timing/study design, were seemingly pre-determined to be immaterial and confirmatory data irrelevant. (Of note, after the New England Journal of Medicine and Lancet studies were retracted, the previously halted WHO studies that were able to resume, did.)
While there was an exuberant and highly publicized effort to denigrate Trump’s HCQ initiative, there was little to no effort to critically evaluate what was being said, correct the records, or communicate the objectively wrong quotes and narratives regarding HCQ following article retractions or as new data emerged contradicting old data.
After the Government/Medical Journals/Media Failed, So Did Hospitals and Academia:
Covid-19 was a fundamental test of medical science, but instead of taking the time to slow down decisions and to stop, focus, and rely on objective, scientific, and clinical fundamentals, America’s trusted federal officials, physicians, and scientists panicked and instead enthusiastically leapt to expensive, novel “warp-speed” solutions on the advice of commercial, for-profit companies. Shockingly few pushed back, and those few that did were silenced. That behavior loosely aligns with 2019 data showing that 91 percent of medical prescribers reflexively believe that FDA-approved products are completely safe and always benefit patients.
As has been made clear with novel Big Pharma Covid-19 approvals, people can manipulate scientific data to promote a narrative, but continued accumulation of factual research, combined with ancillary findings, and the manipulation eventually becomes increasingly difficult, if not impossible. Scientific truth eventually shines through. Detailed evidence in the form of narrative-contrary epidemiological findings regarding both the safety and efficacy of Covid-19 products eventually became apparent.
The true academic medicine scientists didn’t blindly take marching orders from journalists, the WHO, federal agencies, Anthony Fauci, state governors, or anyone else. We methodologically and objectively examined the data and let the accumulated real-world clinical findings speak for themselves. It’s the objective attitude that every scientist should have followed, instead of rushing to conclusions – pandemic or not – madly grasping at the first, shiny, new Big Pharma thing, and then quickly permitting it upon their patients and fellow countrymen through mandates, employer requirements, or financial motivations.
Physicians and Pharmacists Persecuted for Prescribing and Dispensing HCQ:
Clinicians like myself who advocated for any alternate treatments such as ivermectin or HCQ were mocked online by non-medically- and non-scientifically trained “trusted journalists” and “fact-checkers” as being part of a “right-wing conspiracy.” Anyone who didn’t demure to the Covid-19 mRNA or other Big Pharma Covid-19 treatments and narratives were banned, fired, and blasted as “anti-science” around the world and into the reaches of the stratosphere via the internet. And if that wasn’t outrageous enough, it didn’t end there.
After physicians and pharmacists lost their jobs, their reputations, practices, insurance, finances, licensure, and careers were destroyed. That’s because in many cases, even after losing their jobs, state medical and/or pharmacy boards with broad and vague authority plus seemingly limitless taxpayer-funded budgets initiated legal proceedings against their licensure, cherry-picking the persecution of their “off-label” Covid-19 treatments (including ivermectin and HCQ) when other “off-label” treatments for non-Covid-19 diagnoses were a near-ubiquitous component of almost every medical and pharmacy practice. On top of that, America’s press and “fact-checkers” singled out and sought to embarrass providers through online articles.
Starting in May 2024, Americans learned through a Republican House Judiciary report and Elon Musk’s purchase of Twitter that Facebook, YouTube and Amazon, that a great deal of Covid-19 narrative, punishment, and censorship was directly coordinated by the Biden White House via direct legal threats.
Appallingly, it was America’s White House that forced private companies to censor objective facts on now-proven to be effective repurposed treatments (including HCQ) while simultaneously advocating for and/or mandating the use of novel, expensive Big Pharma treatments, and the press were just tools to advance and reinforce the White House’s censorship.
TIMING MATTERS When It Comes to Successful Hydroxychloroquine Treatment for Covid-19:
Negative data surrounding HCQ seemed to be restricted to the US and other Western countries. HCQ was shown to be useful for preventing initial Covid-19 infection when employed as an early treatment protocol, which was why HCQ (or chloroquine) was adopted for Covid-19 treatment during the initial pandemic period, at least in part, by 42 countries (58 countries when including non-government medical organizations).
Most negative studies cited by the American government, academia, and Big Pharma officials ignored a very basic but critically important pharmacology fundamental: Any antimicrobial pharmacology (including: antibiotic, antifungal, antiviral) is substantially less efficacious when it’s implemented during the late-stages of infections, at which point the rapidly replicating infection would overwhelm an individual. Early/immediate treatment is the clinical standard for the treatment of all viral infections, regardless if the virus is: influenza, cold sores, HIV, or Covid-19. Timing is especially important to consider when treating the elderly/infirm.
Despite that, early treatment was ignored in an abundance of “top-tier” peer-reviewed medical journals, including (once again) America’s New England Journal of Medicine. Two prominent, highly-cited, examples are shown below:
SOLIDARITY New England Journal of Medicine (Bibliography #377)
In June of 2020, the New England Journal of Medicine published a poorly designed SOLIDARITY trial denigrating HCQ. HCQ findings were negative because SOLIDARITY study coordinators employed HCQ late treatment methodology to treat Covid-19 patients, despite early treatment being the clinical standard of care.
SOLIDARITY was an open-label RCT (no placebo control arm) trial which showed: 19% higher mortality (p=0.23). SOLIDARITY used 954 very late stage, critical (64% of patients were already on oxygen/ventilation) patients to administer HCQ. Data showed a spike in HCQ mortality at days 5-7, corresponding to about ~90% of the total excess mortality. Almost all excess mortality in this study originates from those very late-stage ventilated patients administered HCQ. HCQ dosage was also exceedingly high and study coordinators did not appear to adjust doses based on patient weight, meaning that potentially toxic dose concentrations from higher doses may have occurred in patients with lower weights.
The WHO authors refer to a lack of excess mortality in the first few days to suggest a lack of toxicity, but seem to fail to consider the very long (approximately 40-day-long) half-life of HCQ. The pharmacology/pharmacokinetics of chloroquine metabolism are complex, with the half-life increasing with increasing dosage. Additionally, an unspecified percentage of patients were administered the relatively more toxic chloroquine as an alternative to HCQ.
RECOVERY New England Journal of Medicine (Bibliography #383)
RECOVERY (Randomized Evaluation of COVID-19 ThERapY) trial published December of 2020 found no significant benefit for very late stage, (9 days after symptom onset) in already very sick patients since viral replication had already overwhelmed patients. As in the SOLIDARITY trial, late treatment for Covid-19 or other viral infections is not the standard of care.
Negative results could have also been due to toxicity from the unusually high dosage used (9.2g total over 10 days) which had been shown in the past to be associated with an increase in risk. Authors did not report results based on weight, BMI, or comorbid underlying conditions such as diabetes and HCQ should be dosed based on weight. Like SOLIDARITY, authors did not adjust HCQ dosage based on patient weight, meaning that toxicity may have been higher in patients of lower weight. Data showed a spike in HCQ mortality on days 5-8, corresponding to ~85% of the total excess seen at day 28 (a similar spike is seen in the SOLIDARITY trial).
Authors note: “we did not observe excess mortality in the first 2 days of treatment…when early effects of dose-dependent toxicity might be expected” but they failed to consider the high dose used on top of the approximately 1,000 to 1,200 hour half-life of HCQ. Administering a drug on a daily basis with a long half-life means that much higher levels of HCQ will be reached later on, as it accumulates. Additionally, patients in this trial were late treatment and extremely sick (median 9 days post symptoms, 60% requiring oxygen and an additional 17% requiring ventilation/ extracorporeal membrane oxygenation (mechanical oxygenation of the blood, extremely high-risk medical intervention with 50% mortality). An unusually high mortality rate was seen in both arms: 1,561 HCQ patients, 3,155 standard-of-care. An additional breakdown of RECOVERY detailed a significant, lengthy list of RECOVERY methodological inconsistencies. (article was internet “auto-translated” from French)
Hydroxychloroquine Reliably Beneficial in Early Treatment Studies:
Despite early treatment being the standard of treatment, it was systematically ignored by investigators, “top-tier” journal peer reviews, and the press. According to a breakdown study analysis of 39 early treatment studies at c19early.com, they showed a dramatic 66% [range: 54-74%] lower risk. Seventeen of those 39 studies show a 76% [61-85%] lower mortality and 16 studies show a 41% [28-51%] lower incidence of hospitalizations.
Late-treatment implementation was also successful, but less so, with 22% [18-26%] lower risk from 264 studies. Very late treatment tended to be not useful and even harmful, especially at excessive dosages – as would be expected with just about any drugs given at excess doses to late-stage patients of any kind.
To visually illustrate this effect, here are two comparisons detailing early treatment studies (Row 1) versus all HCQ study findings (Row 2). While HCQ is generally associated with favorable outcomes, early treatment studies have the most favorable outcomes, (as is typically the case for other antimicrobial pharmacotherapies). Negative outcomes unquestionably occurred but they were typically the result of treatment delay, diagnosis delay, incorrect dosing/duration, and/or otherwise attempting to treat Covid-19 after viral replication had uncontrolled replication for days.
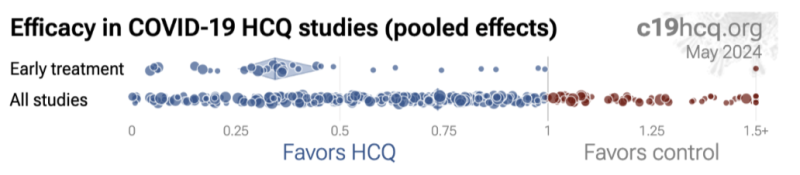

Late Treatment and Incorrect Doses Incorrectly Translated as Hydroxychloroquine Being Harmful and/or “Not Effective:”
Widely disseminated press reports of negative conclusions associated with HCQ reflected the inappropriate timing of HCQ in the form of “late treatment” (or sometimes even very late treatment studies) and/or studies that tended to disregard/not specify an HCQ treatment delay following diagnosis confirmation. The lay press, not knowledgeable in medical pharmacology or treatment standard fundamentals, ignored HCQ timing, dosing, duration, and other important caveats when parroting negative tropes against Trump and his proposals for HCQ. Their narratives originated from what appeared to be coordinated messaging and “top-tier” journals and their expert “peer-reviewers” who in turn seemed to also overlook key issues with HCQ dose, duration, and/or delayed administration.
Well over half of all HCQ clinical studies in the bibliography below ignored the clinical standard of early administration and meeting the definition of less-effective, late treatment – and yet when collectively compiled as part of the meta-analysis, HCQ still demonstrated a somewhat overall (22% [18 26%] lower risk) positive effect.
Specific treatment delays are specified in the full bibliography and their individual accompanying summaries.
General Negative Study Bias in Hydroxychloroquine Publications?
One of the biases from the press and federal officials may have been due to more heavily weighing of seemingly biased studies published in the United States. Curiously, at least one review of data showed that HCQ studies from North America were found to be 2.4 times more likely to report negative results than all studies from the rest of the world, combined.
It showed that the same studies which had negative results associated with HCQ (in late 2020 as Trump was in office promoting HCQ) were correlated with medical/scientific authors having a history of giving donations to the Democratic Party.
That sort of potential study bias is something which would normally warrant vigilant scientific investigation, especially because taxpayer money likely directly or indirectly funded at least some component of almost all American clinical research. Despite that, no investigation has even been proposed, much less carried out.
What about Cochrane’s Review of Hydroxychloroquine?
While Cochrane reviews are and often relied upon reference for the cumulative analysis of many studies, Cochrane HCQ analysis only reviewed 14 studies, (and only analyzed 12 of them). Cochrane’s last review of the data was back in September 2020, ignoring >90% of existing HCQ data. When will Cochrane update its review to include the HUNDREDS of subsequent clinical studies? Who knows, but that is where c19 early analysis comes into play which duplicated Cochrane’s methodology (DerSimonian and Laird random effects model), but expanded it to include all available clinical studies, providing a definitive, up-to-date answer. The full list of the 400+ HCQ clinical studies examined is included in the bibliography.
Along with Cochrane, the press seems to have deliberately ignored a large volume of clinical data both then and now; instead, employing select results to match an anti-HCQ, anti-Trump narrative. HCQ (along with several other repurposed treatments) should have been objectively considered and/or implemented as initial treatments and/or non-placebo alternates, and/or comparators to mRNA technology or against other expensive, novel Covid-19 treatments, including Paxlovid and Remdesivir.
Based on these data, it appears likely that HCQ would have been superior from both a safety and efficacy standpoint – and with Trump’s “donated” supply – free.
Free, Donated Hydroxychloroquine vs Expensive, Novel Remdesivir Study Biases:
While ironically ignoring positive findings of HCQ and implementing early treatment methodology standards ignored with HCQ, remdesivir was approved and endorsed by the FDA for the treatment of Covid-19 based on an April 2020 study that yielded no positive results.
Despite that, the FDA approved remdesivir anyway, and without even consulting their own appointed advisory committee.
A similar expedited approval took place in the EU, just before the disappointing WHO trial data was released, and apparently while the disappointing trial results were known to the manufacturer. Gilead’s aggressive marketing campaign proceeded despite questionable efficacy and the lack of transparency in both the FDA/EU approvals.
The SOLIDARITY trial, which also contained an arm which examined remdesivir, showed that remdesivir did not reduce mortality or decrease the time that it took for patients to recover from Covid-19.
The preponderance of the cumulative, up-to-date findings illustrates that there are no statistically significant or clinically meaningful improvements with remdesivir use.
In the few positive studies, the small non-significant mortality improvement disappears with longer follow-up duration. Despite all of that, hospitals were financially incentivized to entice patients to use remdesivir by granting a mysterious 20% “boost” bonus payment from Medicare on the entire hospital bill for those patients who agreed to receiving remdesivir and larger bonus payment to the hospital if a Covid-19 patient is mechanically ventilated. In the end, that worked out to each hospital receiving “at least” a $100,000 “bonus” per patient, paid for by American tax dollars.
Unlike fully transparent and available HCQ studies for anyone to examine, Remdesivir study data and official messaging has been described by scientists as confusing, unfair, incomplete, and untransparent. Study findings detailed 8.6% more deaths in the Remdesivir group than in the placebo group. The results of that study, showed on day 28, 7.2% (22 out of 158) in the Remdesivir arm died, while 7.8% (10 out of 78) in the placebo arm died.
Remdesivir studies also had the metric of “death” removed from its primary endpoint in what is now a familiar pattern of the FDA failing to warn Americans about adverse events while permitting Big Pharma to skew drug safety data collection methodology by simply not requiring collecting it. The difference in death rate, one of the original primary measures, was not statistically significant, showing only a marginal reduction from 11 percent in patients given a placebo to 8 percent in patients given remdesivir.
The available clinical data did not support remdesivir approval, let alone a strong White House endorsement, let alone a federally sanctioned payment incentive for hospitals, from taxpayers. The confusion regarding both the approval of and the financial incentive borne by taxpayers perplexed enough people to the point that it was even criticized in non-medical publications such as science.org.
Bottom line: Remdisivir was (probably) not safe and not effective, even though it was tested against “early treatment” patient parameters, in contrast to HCQ studies, and still showed negative findings. It was then expedited for approval by FDA officials and medical reviewers. Unlike Trump’s donated HCQ which had transparently positive evidence for benefit in early treatment, Remdisivir had extremely limited clinical history (versus HCQ’s 55 years of clinical history), was not efficacious, and less safe, and because of some unclear deal, had a significant financial incentive encouraging its administration, in turn making hospital bills substantially more expensive.
Four Years Later: Persistent, Incorrect, 2024 Claims of “Hydroxychloroquine Doesn’t Work for Covid-19”
As of this very day, there are some major medical centers, medical schools, and other organizations that still have operating webpages, very conspicuously appearing in the first page of internet search results, actively continuing to advocate for remdesivir while simultaneously regurgitating incorrect narratives about how HCQ should not be used for Covid-19.
All of the below results showed up very prominently (on the first page of results) following a routine internet search for the facility name and the terms “hydroxychloroquine covid” during July 2024.
Despite an abundance of objective, real-world data fundamentally stating otherwise for years now, for many people the Big Pharma/White House narrative scales will never fall from their eyes. Indeed, there are none so blind as those who will not…examine the clinical evidence and compare assessment methodologies.
Specifically: here is a list of major medical centers that are still parroting outdated, incorrect tropes regarding HCQ. Although all of the website statements below are incorrect, some are “more incorrect” than others as Orwell might have said. Here are just a selection:
- Mayo Clinic: “[HCQ] is not recommended as a treatment for coronavirus disease… Also, hydroxychloroquine doesn’t prevent infection with the virus that causes COVID-19.”
- Cochrane Policy Institute: “[HCQ] does not reduce deaths from COVID-19…”
- Drugs.com: “[HCQ] does not provide a medical benefit for hospitalized patients with COVID.”
- Honor Health Hospital Network: “Question: Should I have [HCQ] on hand in case I feel sick?… Answer: No.”
- Cleveland clinic: “Overall, [HCQ]…has never been shown to be helpful in fighting COVID-19.”
- Duke University Medical Center: “Hydroxychloroquine is the most disappointing, disavowed drug that researchers keep studying for COVID-19.”
- Baylor University Medical Center: “Randomized controlled trials have repeatedly shown that [HCQ] is not effective to treat or prevent COVID-19.”
- Baylor’s Dr. Peter Hotez: “Ivermectin does nothing to help people with Covid, same with [HCQ].”
- Kaiser Permanente Health: “[HCQ] is not recommended for coronavirus infection…unless you are enrolled in a study.”
- Houston Methodist Hospital who suspended HCQ advocate Dr. Mary Bowden: “[her] opinions are harmful to the community, do not reflect reliable medical evidence or the values of Houston Methodist.” Hospital spokesman went on to say Dr. Bowden was ‘spreading dangerous misinformation not based in science.’”
- U.S. Food and Drug Administration: “[HCQ has] not been shown to be safe and effective for treating or preventing COVID-19.”
The FDA link immediately above goes on to reiterate the established cardiac adverse events and drug-drug interactions associated with HCQ use, as per its safety review memorandum discussed earlier.
Abundance of Scientific Data Shows Safety and Effectiveness of HCQ for Covid-19:
Much like two consecutive Cochrane-reviewed scientific studies showing that all widely-mandated masks, including surgical masks, and N-/KN-95 masks are almost certainly ineffective for inhibiting Covid-19 transmission, study data trickled out over time about the benefits of HCQ soon after Covid-19 started spreading. Those findings eventually accumulated into the avalanche of clinical evidence before us today, illustrating that HCQ is objectively effective for Covid-19 prevention and treatment. It is no exaggeration to say that HCQ would have helped many millions.
To clarify the evidence, while I have a scattered mess of HCQ studies on my computer, office and bedroom, dog-eared and food-stained, there is an elegantly-presented meta-analysis which employs the same analytical methodology that Cochrane reviews use. It details how the compilation of: over 400 studies, conducted by over 8,000 scientists, involving over 525,000 patients across 58 countries, showed that the appropriate clinical utility of HCQ for Covid-19 resulted in a statistically significant lower risk for 1) mortality, 2) hospitalization, 3) recovery, 4) cases, and 5) viral clearance.
Of note, this was not just a cherry-picking slash Texas sharpshooter fallacy of select data findings; it represents a compilation of all available clinical data.
Reviewing and Evaluating All Available Clinical Studies:
In composing an argument, one needs to consider all available legitimate data – not just select summary findings parroted by news reporters or exclusively relying on findings from selected “top-tier” medical journals. It is no secret that “top-tier” journals accept significant sponsorship from Big Pharma to cover its expenses, which now include ancillary, non-sequitur agendas such as an experimental “artificial intelligence division.” As has become clear in the recent decade, and most apparent under Covid-19, studies published in “top-tier” journals including the New England Journal of Medicine, The Journal of the American Medical Association and the Lancet are not Holy Scripture above critique, and can actually be dead wrong.
That’s why it’s important to get corroboration from alternate sources. There are very legitimate clinical data being published, including data from other countries and/or data published in smaller journals (without Big Pharma sponsorship) worthy of clinical and epidemiological consideration. In fact, academics who spend their lives in medical research will tell you that non-big-name, smaller studies, observational, and/or real-world study data when examined in combination are not only very worthy of consideration – but may be more reflective of a drug’s efficacy and safety. In other words, the totality of evidence from multiple, well-designed, and smaller, real-world, case reports, case series, and/or observational trials can actually be a stronger indicator of a clinical/statistical effect than that of just one or a few biased large trials.
To-date there are over 400 clinical studies examining HCQ use in Covid-19 with both negative and positive findings. A complete bibliography of all studies, and study summaries examined are provided in the form of an annotated bibliography at the end of this article.
The list and review of the data and the bibliography excluded studies known to be the product of fraudulent research, including those by Elshafie, Dabbous#1, Dabbous#2, Abd-El-Salam, and the aforementioned Desai Lancet and New England Journal of Medicine publications.
The Good and Bad of Large Randomized Controlled Trials (RCTs):
Randomized Controlled Trials (RCTs) are conceptually preferred if they are properly designed and conducted. However, the Covid-19 era exposed critical biases in such trials, including but not limited to: treatment delays (any antiviral treatment for any viral infection, including Covid-19 must begin promptly), protocols that were designed to fail, mid-study changes to the study protocol, biased analysis and presentation, lack of transparency in data, and suspiciously timed publication releases.
As has been shown here, biases on top of important study design and standard-of-care treatment shortcomings can lead to wildly incorrect clinical study conclusions. Every HCQ clinical study needs to be evaluated on individual merit for potential biases and/or confoundings, whether randomized, real-world, observational, large, or small trials.
Large RCTs allegedly producing the Big Pharma-generated-idiom of “Evidence Based Medicine” published in “top-tier” journals often appear very compelling – especially because they are what the lay press tends to focus on – but in the recent past, it has become clear that responsible clinical scientists must very carefully examine methodology used beyond the high-level summary overviews, and to also look at additional non-RCT sources of data for confirmation of findings.
Another problem with large RCTs is that unlike real-word and observational studies, not just anyone can conduct large RCTs. Barriers include them often being significantly more expensive, time-consuming, and requiring a dedicated, highly skilled support staff. That prevents less-well-funded clinicians who have smaller practices/facilities or clinicians that have employment requirements which have a focus on direct care responsibilities as opposed to clinical research.
While federal grants are available for RCT endeavors, those grants are highly competitive and tend to be limited to particular diseases or topics which in turn end up being awarded to particular facilities with the aforementioned support staff, infrastructure, et cetera. Those major centers and/or their employees tend to be connected in one way or another to Big Pharma funding.
When Covid-19 emerged, billions of taxpayer dollars were given to Big Pharma. That kind of trust seems to have beguiled unethical physicians and scientists to create an incentive to show a lack of effectiveness or safety for inexpensive generic products, while in turn show efficacy for a novel, expensive, patented commercial product as a way for obtaining even more taxpayer money. Big Pharma scientists can be motivated to show benefit to their product versus existing, less expensive, or generically available technology. This scenario not only applies to Covid-19 treatments such as HCQ or ivermectin, but to a fair amount of all investigational medicine research.
These sorts of biases can lead to discordant findings in RCT versus real-world clinical findings. As is the case with discordant HCQ findings, and/or other studies related to Covid-19 approvals, it’s important to investigate the reasons as to why. Unfortunately, it appears that there is little to no FDA/CDC/NIH or White House appetite for unearthing the truth. The evidence shown here is a preliminary concept into what an investigation ought to examine.
You Can Run (With a False Narrative) but You Can’t Hide (From the Data): Analyzing All Available Hydroxychloroquine Clinical Data:
A meta-analysis combines studies to perform a broad analysis. This type of methodology is accurate, valid, and widely respected by epidemiologists, statisticians, and other medical/scientific disciplines. In fact, the present analysis of HCQ in the bibliography here employs the same analysis methodology that Cochrane routinely uses to provide a complete picture of an effect across studies.
These statistical findings from clinical studies are in addition to the highly plausible molecular biology and pharmacological mechanisms of how HCQ is effective for preventing the entry of many viruses into cells. For purposes of keeping the length of this review manageable, the pharmacologic mechanism of action of HCQ will not be discussed here.
Comprehensive Meta-Analyses Evaluating all Findings (Both Good and Bad) Show Hydroxychloroquine’s Benefit:
Meta-analyses which combine RCT and observational/real-world-use studies across many facilities make the strongest case. The dependence on any individual trial is subject to potential confounding, shortcomings, errors, bias, incompetence, and even fraud.
A diagram adapted from a Nature publication below illustrates a scenario in which four smaller studies that may not have delivered statistical significance individually (ie, have a p>0.05), but could show strong evidence with a statistical significance when analyzed in combination via a meta-analysis:
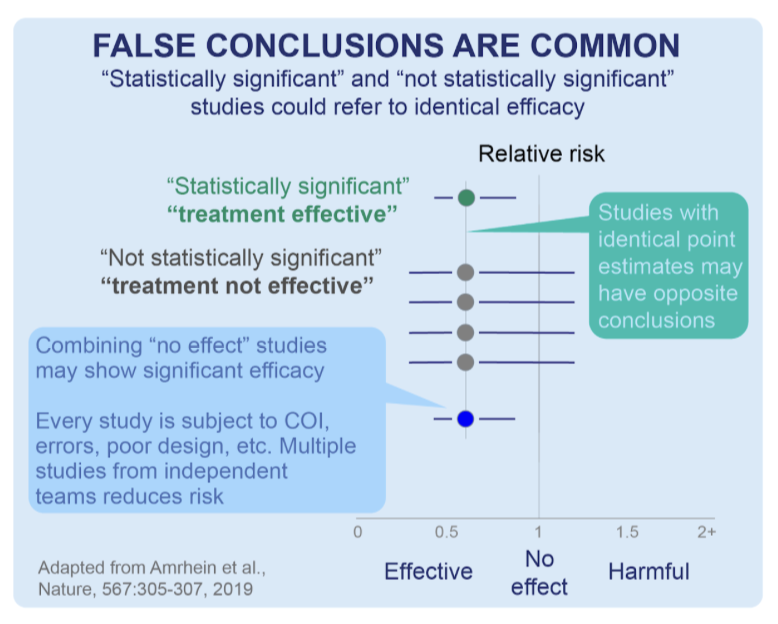

To date, the DerSimonian and by the random effects meta-analysis model conducted by the c19early analysts shows a clinically beneficial effect of HCQ for Covid-19 treatment with a certainty of p<0.00000000001 (that is, one in one sextillion) over all >400 HCQ studies.
RCTs for specific outcomes like mortality, hospitalization, and recoveries each show a very strong benefit with a p<0.0001.
The beneficial effect of HCQ includes delayed treatment and other negative result studies, despite late treatment being significantly less effective. Treatment delay and/or late (and sometimes very late) treatment was implemented in over half (n=264) of the HCQ studies in the bibliography below. Of note, a high number of late/very late/delayed treatment studies compiled into the meta-analysis still ended up showing some beneficial effect of HCQ administration, illustrating its strong efficacy. Potential confounding factors may include viral replication, viral loading dose, viral variant/mutation, on top of numerous demographic, immunologic, and other factors. Avoiding treatment delay is a fundamental concept taught early on in both pharmacy and medical schools.
Financial Ramifications of Condemning Trump’s Hydroxychloroquine Proposals in Favor of Big Pharma Alternatives:
While Trump’s proposal to use HCQ was negatively bombarded, novel, expensive Big Pharma treatments with very limited data were developed, (and tested against placebo instead of treatments including HCQ or ivermectin) and rapidly reviewed, authorized by America’s FDA and purchased with taxpayer debt by the Biden White House. Despite limited findings, Paxlovid ($1,400 per treatment course), Remdisivir ($3,120 per course), and Molnupiravir ($700 per course) were White House-endorsed despite Trump having already secured HCQ for free. By the end of 2021 alone, the White House had already spent over $10.6 billion just on Paxlovid alone and subsequently purchased more. All of the White House’s Covid-19 treatments were lacking long-term efficacy/safety findings as compared to HCQ.
For perspective: the greater than $10.6 billion the government spent on Paxlovid just through 2021 alone could have instead purchased about 353,000 $30,000 Toyota Camry SEs (the most popular model) for destitute Americans who lost their cars due to the economic downturn.
Even worse: according to the most recent findings, (and like remdesivir) Paxlovid doesn’t work, even if you double the length of dosing according to the most recent and cumulative findings published in the July 2024 issue of the New England Journal of Medicine.
It reconfirms an earlier published case report just weeks after Paxlovid was approved and given to Americans showing that people who take Paxlovid don’t get better sooner, compared to those taking placebo. The medical community has known, and written about Paxlovid rebound which occurred from the very beginning.
Of note, rebound from HCQ is significantly less likely to occur because of its very long, aforementioned half-life.
Today, even the White House-fawning press is openly mocking the use of Paxlovid for Joe Biden’s July 2024 infection with Covid-19 in both the title and photo caption from Business Insider below:


Between $16 and $22 Trillion Wasted:
Trump’s donated HCQ for pre-exposure prophylaxis, early exposure, or early treatment (in eligible individuals), would have worked better than Paxlovid and could have also been used to prevent the numerous strains of Covid-19 from the beginning.
And the tens of billions of dollars wasted on Paxlovid and other Big Pharma boondoggles were chicken scratch relative to the entire cost of the pandemic.
It’s been estimated that the Covid-19 pandemic has cost Americans at least $16 trillion according to Harvard economic researchers, $18 trillion according to Heritage Foundation scholars, with other estimates being even higher from the Institute for Progress. It’s hard to imagine how much even $1trillion is, but here is one example relative to seconds or days. Relative to automobiles, using Harvard’s lowest estimate of $16 trillion, that amount of money could have instead bought a new $30,000 Toyota Camry SE for every single American citizen (man, woman and child of any age) in America with over $5 trillion left over. Instead, Americans are not only not getting a new Toyota Camry SE, but are instead losing the cars they do have, losing their homes, and are otherwise being crushed by high inflation on just about everything they need, including food, gasoline, baby formula, and electricity.
It is no exaggeration to state that Trump’s HCQ proposal could have prevented much of the negative financial, social, and psychiatric ramifications of Covid-19 – not to mention morbidity and mortality. According to the meta-analysis of the studies in the bibliography below, HCQ would have been effective and could have potentially avoided the vast majority of its $16 trillion expenditure.
The bottom line is: President Trump was correct to secure a donation of, and advocate for the use HCQ for eligible individuals. The most recent cumulative positive findings associated with HCQ are undeniable evidence that Americans would have been better off had HCQ had been implemented and used in eligible populations.
Hydroxychloroquine Summary Data Graphs:
To fully address transparency, I am including a full list of HCQ studies completed to date which comprise the meta-analysis showing HCQ’s efficacy and safety. Each of the 400-plus references include a brief summary and a link to a longer analysis at c19early.
The bibliography includes all clinical data including both positive and negative findings that implemented wrong dosing, too-short-duration, and studies which employed late treatments. It also included studies not reaching statistical significance (p>0.05). Hyperlinks to the original studies data are also provided.
In some cases, journals held clinical studies from publication for an extended period and then rejected papers without review (some journals still refuse to defy Big Pharma sponsors and/or may have been threatened to censor their data by order of the White House). For others, authors could have lost authorization from their employer to publish, or they may no longer want to pursue journal publication for fear of negative impacts on their careers or personal or employer funding. It’s also a distinct possibility that some authors simply deprioritized advancing publishing and moved on to other research or clinical duties required of them when Covid-19 morbidity and mortality collapsed following emergence of the Omicron variant (in late 2021) along with the downstream variants thereof.
Along with the bibliography, I am also including several HCQ scatter plots illustrating the number of negative versus positive findings from c19early on overall benefit, and breakdown of relative benefits from prophylaxis, early and late treatments.
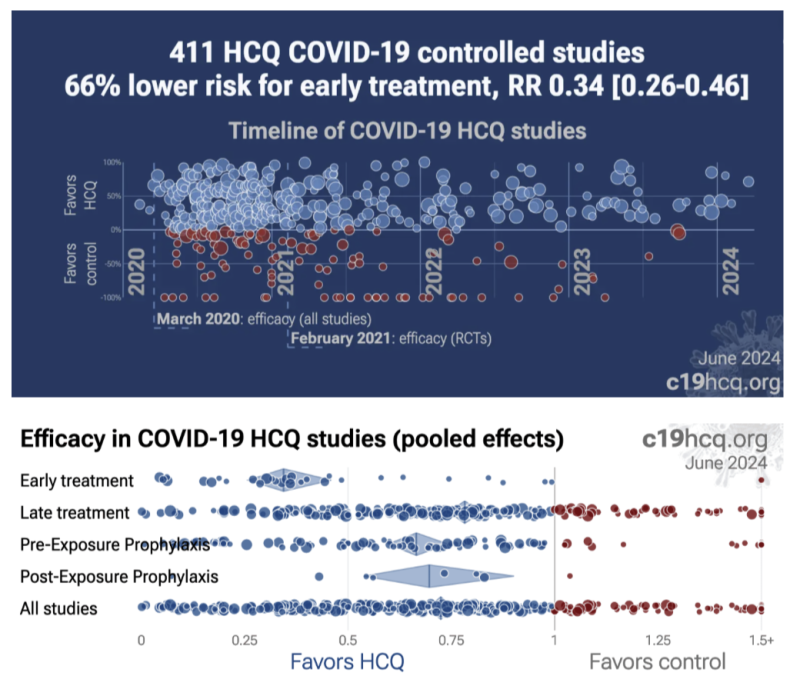

Summary:
“Tip-tier” medical journals, mainstream press, hospitals, administrators, insurance companies, Big Pharma, state/municipal government, on top of every federal alphabet agency and others all converged to a singularity of promoting a grand illusion of manufactured consent that demonized HCQ while favoring novel, minimally tested, minimally effective, expensive commercial Covid-19 treatments. The linked meta-analysis now proves HCQ’s effectiveness. It’s no exaggeration to state that the manipulation of data that occurred with HCQ (and other repurposed drug treatments) was the biggest scandal in the history of American medicine, and one of the biggest medical crimes against humanity.
The mission of science and scientists is to cultivate critical thinking coupled with a willingness of its disciples to adjust their thinking and admit being wrong about existing ideas or theories. In other words, recognizing that no science is ever entirely “settled” and therefore should not be silenced.
- Confirmation bias in referencing select HCQ RCTs was not science.
- The FDA’s safety memorandum on HCQ involved inappropriate, cherry-picked data and was not science.
- Ignoring the standard of care and mostly considering late and very late and inappropriate doses/durations of HCQ treatments as a means to denigrate Trump’s HCQ proposal was not science.
- Silencing medical and scientific experts critical of the White House, FDA, and Big Pharma was not science.
- The lay press’s incompetent evaluation and parroting of highly flawed HCQ data was not science.
- Articles from The Hill, Forbes’s, and Politico which rushed to amplify critique on HCQ and Trump – but following study retraction, keep their articles online and continually accessed by the public, was not science.
- Medical journals not demanding lay-press corrections on updated/retracted findings based on its internal “peer-review” failures to verify non-sequitur HCQ findings was not science.
- Hospital narratives regarding HCQ published on public-facing websites was not science.
- The failure of “peer reviews” at “top-tier” medical journals to consider established clinical treatment standards of early treatment was not science.
- Allowing non-medically trained “fact-checkers” to comment on medically and technically complex aspects of pharmacology and medicine was not science.
- Punishing community pharmacists and physicians for appropriately choosing to dispense and prescribe HCQ for Covid-19 was not science.
- Demanding a single “consensus” on how pharmacists and physicians were permitted to treat Covid-19 was not science.
- A federal official (or any one person) referring to himself uniquely as “the science” was not science.
Thousands of well-educated scientists and clinicians in America’s federal government, universities, and hospital settings ignored the historical, careful scientific evaluation process established over millennia by their scientific predecessors. No falsehood regarding HCQ for Covid-19 was too great, and every distortion of the truth was justified as necessary, not only to destroy HCQ, but Donald Trump’s mere well-intended recommendation of using HCQ for eligible patients.
This is how the anti-HCQ narrative was created. Everyone in charge seemed to mysteriously unify themselves in a coordinated “consensus” against HCQ.
In place of HCQ, new, minimally tested, expensive, extremely complex, rarely used gene therapy technology was proposed by Big Pharma, then unscientifically mandated by the Biden White House, and funded with obscene debt. The lies about HCQ and other repurposed treatments like ivermectin were promoted by the government and news organizations, making it seem like mRNA injections, unlimited boosters and novel, FDA-sanctioned therapies were only acceptable ways to prevent or treat Covid-19. The end product of the falsehoods and mRNA mandates adversely affected every single American citizen, with a very select few reaping astonishing profits, borne on the back of taxpayers.
Donald Trump and RFK’s recent collaboration and collaboration to Make America Healthy Again (MAHA) should eventually include a full investigation into blockaded HCQ as a repurposed treatment for Covid-19 in order to better understand its seemingly coordinated and wholly inappropriate condemnation.
It is no exaggeration to state that had Trump been allowed to proceed with his endeavor to distribute HCQ for Covid-19 in an appropriate patient population, we would be living in a much different United States of America. Today’s cumulative safety and efficacy findings on HCQ data are unequivocal proof detailing its benefits, particularly for the early treatment of Covid-19.
DISCLAIMER: Do NOT discontinue or initiate taking ANY drug without first discussing it with a pharmacist or physician you know and trust.
Dr. David Gortler is a pharmacologist and pharmacist. He is a former Yale University School of Medicine professor of pharmacology and biotechnology. While at Yale, he was recruited by the FDA to become a medical officer/senior medical analyst in the FDA’s Office of New Drugs. He was later appointed as senior advisor to the FDA commissioner on drug safety and FDA science policy. He is currently a senior fellow at the Heritage Foundation in Washington, DC, having previously served as a fellow at the Ethics and Public Policy Center.
Heritage is listed for identification purposes only. The views expressed in this article are the author’s own and do not reflect any institutional position for Heritage or its Board of Trustees.
Bibliography
1. Y. Su, Y. Ling, Y. Ma, L. Tao, Q. Miao, Q. Shi, J. Pan, H. Lu, and B. Hu, Efficacy of early hydroxychloroquine treatment in preventing COVID-19 pneumonia aggravation, the experience from Shanghai, China Dec 2020, BioScience Trends, Volume 14, Issue 6, Page 408-414
EARLY TREATMENT HCQ early treatment study: 85% lower progression (p=0.006), 24% faster improvement (p=0.02), and 36% improved viral clearance (p=0.001).
85% lower disease progression with early use of HCQ. Retrospective 616 patients in China showing adjusted progression, hazard ratio 0.15, p = 0.006. https://c19p.org/su
2. Purwati, Budiono, B. Rachman, Yulistiani, A. Miatmoko, Nasronudin, S. Lardo, Y. Purnama, M. Laely, I. Rochmad, T. Ismail, S. Wulandari, D. Setyawan, A. Rosyid, H. Setiawan, P. Wulaningrum, T. Asmarawati, E. Marfiani, S. Yuniati, M. Fuadi, P. Endraswari, Purwaningsih, E. Hendrianto, D. Karsari, A. Dinaryanti, N. Ertanti, I. Ihsan, D. Purnama, and Y. Indrayani, A Randomized, Double-Blind, Multicenter Clinical Study Comparing the Efficacy and Safety of a Drug Combination of Lopinavir/Ritonavir-Azithromycin, Lopinavir/Ritonavir-Doxycycline, and Azithromycin-Hydroxychloroquine for Patients Diagnosed with Mild to Moderate COVID-19 Infections Feb 2021, Biochemistry Research Int., Volume 2021, Page 1-12
LATE TREATMENT 754 patient HCQ late treatment RCT: 66% improved viral clearance (p<0.0001).
RCT 754 patients comparing HCQ+AZ along with other treatment groups using lopinavir/ritonavir and doxycycline to a control group taking AZ, finding significantly faster viral clearance with all treatment groups. (Note: The labels in Figure 2 appear to be reversed). https://c19p.org/purwati
3. T. Sulaiman, A. Mohana, L. Alawdah, N. Mahmoud, M. Hassanein, T. Wani, A. Alfaifi, E. Alenazi, N. Radwan, N. AlKhalifah, E. Elkady, M. Alanazi, M. Alqahtani, K. Abdullah, Y. Yousif, F. AboGazalah, F. Awwad, K. Alabdulkareem, F. AlGhofaili, A. AlJedai, H. Jokhdar, and F. Alrabiah, The Effect of Early Hydroxychloroquine-based Therapy in COVID-19 Patients in Ambulatory Care Settings: A Nationwide Prospective Cohort Study Sep 2020, medRxiv
EARLY TREATMENT 7,892 patient HCQ early treatment study: 64% lower mortality (p=0.01), 44% lower combined mortality/ICU admission (p=0.02), 37% lower ICU admission (p=0.13), and 39% lower hospitalization (p<0.0001).
Observational prospective 5,541 patients, adjusted HCQ mortality odds ratio OR 0.36, p = 0.012. Adjusted hospitalization OR 0.57, p < 0.001. Zinc supplementation was used in all cases. Early treatment in ambulatory fever clinics in Saudi Arabia. https://c19p.org/sulaiman
4. R. Seet, A. Quek, D. Ooi, S. Sengupta, S. Lakshminarasappa, C. Koo, J. So, B. Goh, K. Loh, D. Fisher, H. Teoh, J. Sun, A. Cook, P. Tambyah, and M. Hartman, Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: an open-label randomized trial Apr 2021, Int. J. Infectious Diseases, Volume 106, Page 314-322
1,051 patient HCQ prophylaxis RCT: 35% fewer symptomatic cases (p=0.05) and 32% fewer cases (p=0.009).
Prophylaxis RCT in Singapore with 3,037 low risk patients, showing lower serious cases, lower symptomatic cases, and lower confirmed cases of Covid-19 with all treatments (ivermectin, HCQ, PVP-I, and Zinc + vitamin C) compared to vitamin C. Only 71.4% reported >70% adherence, limiting efficacy. QTc did not statistically significantly differ between baseline and follow-up readings (mean 379 vs 378ms, paired t-test p=0.387). Meta-analysis of vitamin C in 6 previous trials shows a benefit of 16%, so the actual benefit of ivermectin, HCQ, and PVP-I may be higher. Cluster RCT with 40 clusters. There were no hospitalizations and no deaths. https://c19p.org/seeth
5. I. Simova, T. Vekov, J. Krasnaliev, V. Kornovski, and P. Bozhinov, Hydroxychloroquine for prophylaxis and treatment of COVID-19 in health care workers Nov 2020, New Microbes and New Infections, Volume 38, Page 100813
EARLY TREATMENT 38 patient HCQ early treatment study: 94% lower hospitalization (p=0.01) and 96% improved viral clearance (p=0.001).
100% reduction in hospitalization and cases with early treatment using HCQ+AZ+zinc. Brief report on healthcare workers in Bulgaria. 0 hospitalizations with treatment vs. 2 for control 0 PCR+ at day 14 with treatment vs. 3 for control 33 treatment patients and 5 control patients. No serious adverse events. This paper reports on both PEP and early treatment, we have separated the two studies. https://c19p.org/simova
6. H. Tsanovska, I. Simova, V. Genov, T. Kundurzhiev, J. Krasnaliev, V. Kornovski, N. Dimitrov, and T. Vekov, Hydroxychloroquine (HCQ) treatment for hospitalized patients with COVID-19 Mar 2022, Infectious Disorders – Drug Targets, Volume 22
LATE TREATMENT 140 patient HCQ late treatment PSM study: 58% lower mortality (p=0.03), 74% lower ventilation (p=0.0007), and 70% lower ICU admission (p=0.0004).
PSM prospective study of 260 Covid-19 patients in Bulgaria, showing lower mortality, ventilation, and ICU admission with HCQ treatment. https://c19p.org/tsanovska
7. B. Yu, C. Li, P. Chen, J. Li, H. Jiang, and D. Wang, Beneficial effects exerted by hydroxychloroquine in treating COVID-19 patients via protecting multiple organs Aug 2020, Science China Life Sciences, 2020 Aug 3, Volume 64, Issue 2, Page 330-333
LATE TREATMENT 2,882 patient HCQ late treatment study: 83% lower progression (p=0.05) and 85% lower mortality (p=0.02).
Retrospective 2,882 patients in China, median age 62, 278 receiving HCQ, median 10 days post-hospitalization, showing that HCQ treatment can reduce systemic inflammation and inhibit the cytokine storm, thus protecting multiple organs from inflammatory injuries, such as detoxification in the liver and attenuation of cardiac injury. IL-6 levels significantly reduced after HCQ treatment (p<0.05). The significantly lower dose used here is potentially related to the different observations from the RECOVERY trial results. Authors suggest that treatment should be started as soon as possible. The 550 patients that were critically ill at baseline are reported in a separate paper. For the non-critically-ill patients at baseline, the proportion of patients that became critically ill was significantly lower for those treated with HCQ. For the subset of patients that started HCQ treatment early only 1.4% died versus 3.9% for HCQ started late and 9.1% for control patients. https://c19p.org/yu2
8. K. Hong, J. Jang, J. Hur, J. Lee, H. Kim, W. Lee, and J. Ahn, Early Hydroxychloroquine Administration for Rapid Severe Acute Respiratory Syndrome Coronavirus 2 Eradication Jul 2020, Infect. Chemother., 2020, Volume 52, Issue 3, Page 396
EARLY TREATMENT 90 patient HCQ early treatment study: 65% improved viral clearance (p=0.001).
HCQ 1-4 days from diagnosis was the only protective factor against prolonged viral shedding found, OR 0.111, p=0.001. 57.1% viral clearance with 1-4 days delay vs. 22.9% for 5+ days delayed treatment. Authors report that early administration of HCQ significantly ameliorates inflammatory cytokine secretion and that COVID-19 patients should be administrated HCQ as soon as possible. 42 patients with HCQ 1-4 days from diagnosis, 48 with HCQ 5+ days from diagnosis. https://c19p.org/hong
9. Z. Chen, J. Hu, Z. Zhang, S. Jiang, S. Han, D. Yan, R. Zhuang, B. Hu, and Z. Zhang, Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial Mar 2020, medRxiv doi:10.1101/2020.03.22.20040758
LATE TREATMENT 62 patient HCQ late treatment RCT: 57% lower pneumonia (p=0.04).
62 patients. RCT showing significantly faster recovery with HCQ. 13% progressed to severe cases in the control group, versus 0% for the treatment group. Significant improvement seen in pneumonia on chest CT for 61% of treated patients and 16% of control patients. https://c19p.org/chenrct
10. G. Reis, E. Moreira Silva, D. Medeiros Silva, L. Thabane, G. Singh, J. Park, J. Forrest, O. Harari, C. Quirino dos Santos, A. Guimarães de Almeida, A. Figueiredo Neto, L. Savassi, A. Milagres, M. Teixeira, M. Simplicio, L. Ribeiro, R. Oliveira, and E. Mills, Effect of Early Treatment With Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients With COVID-19 The TOGETHER Randomized Clinical Trial Apr 2021, JAMA Network Open, Volume 4, Issue 4, Page e216468
LATE TREATMENT 441 patient HCQ late treatment RCT: 24% lower hospitalization (p=0.57) and 4% improved viral clearance (p=0.1).
Early terminated RCT in Brazil showing lower mortality and hospitalization with HCQ, but not reaching statistical significance. Although the title includes “early treatment” that treatment was relatively late, with most patients being over 5 days from the onset of symptoms. Adverse events were lower in the HCQ group compared to the control group. This trial appears to have been terminated at 45% enrollment while showing ≥70% probability of superiority. The futility threshold was not reported, but it would be highly unusual for it to be as high as 70%. The paper indicates the placebo was talc,; however the trial protocol shows the “placebo” as vitamin C, for which there are 7 Covid-19 treatment studies as of April 2021 that collectively show significant efficacy. Results differ significantly from those reported prior to publication. Prior to publication, authors reported an RR for hospitalization or death of 1.0 [0.45-2.21]. https://c19p.org/reis
11. M. Million, J. Lagier, H. Tissot-Dupont, I. Ravaux, C. Dhiver, C. Tomei, N. Cassir, L. Delorme, S. Cortaredona, S. Amrane, C. Aubry, K. Bendamardji, C. Berenger, B. Doudier, S. Edouard, M. Hocquart, M. Mailhe, C. Porcheto, P. Seng, C. Triquet, S. Gentile, E. Jouve, A. Giraud-Gatineau, H. Chaudet, L. Camoin-Jau, P. Colson, P. Gautret, P. Fournier, B. Maille, J. Deharo, P. Habert, J. Gaubert, A. Jacquier, S. Honore, K. Guillon-Lorvellec, Y. Obadia, P. Parola, P. Brouqui, and D. Raoult, Early Treatment with Hydroxychloroquine and Azithromycin in 10,429 COVID-19 Outpatients: A Monocentric Retrospective Cohort Study May 2021, Reviews in Cardiovascular Medicine, Volume 22, Issue 3, Page 1063
EARLY TREATMENT 10,429 patient HCQ early treatment study: 83% lower mortality (p=0.0007), 44% lower ICU admission (p=0.18), and 4% lower hospitalization (p=0.77).
Retrospective 10,429 outpatients in France, 8,315 treated with HCQ+AZ a median of 4 days from symptom onset, showing significantly lower mortality with treatment. https://c19p.org/million4
12. L. Chen, Z. Zhang, J. Fu, Z. Feng, S. Zhang, Q. Han, X. Zhang, X. Xiao, H. Chen, L. Liu, X. Chen, Y. Lan, D. Zhong, L. Hu, J. Wang, X. Yu, D. She, Y. Zhu, and Z. Yin, Efficacy and safety of chloroquine or hydroxychloroquine in moderate type of COVID-19: a prospective open-label randomized controlled study Jun 2020, medRxiv
LATE TREATMENT 48 patient HCQ late treatment RCT: 20% faster recovery (p=0.51) and 71% faster viral clearance (p=0.0004).
RCT 48 hospitalized patients in China showing faster clinical recovery and viral clearance with CQ/HCQ. https://c19p.org/chen
13. A. Vaezi, E. Nasri, H. Fakhim, M. Salahi, S. Ghafel, S. Pourajam, A. Darakhshandeh, N. Kassaian, S. Sadeghi, B. Ataei, and S. Javanmard, Efficacy of hydroxychloroquine in pre-exposure severe acute respiratory syndrome coronavirus 2 prophylaxis among high-risk healthcare workers: A multicenter study Jan 2023, Advanced Biomedical Research, Volume 12, Issue 1, Page 3
143 patient HCQ prophylaxis RCT: 92% fewer symptomatic cases (p=0.03).
RCT 143 healthcare workers in Iran, showing lower cases with HCQ prophylaxis, statistically significant only for moderate/severe cases. Baseline details are not provided. https://c19p.org/nasri
14. T. Rouamba, E. Ouédraogo, H. Barry, N. Yaméogo, A. Sondo, R. Boly, J. Zoungrana, A. Ouédraogo, M. Tahita, A. Poda, A. Diendéré, A. Ouedraogo, I. Valea, I. Traoré, Z. Tarnagda, M. Drabo, and H. Tinto, Assessment of Recovery Time, Worsening and Death, among COVID-19 inpatients and outpatients, under treatment with Hydroxychloroquine or Chloroquine plus Azithromycin Combination in Burkina Faso Feb 2022, Int. J. Infectious Diseases
LATE TREATMENT 864 patient HCQ late treatment study: 80% lower mortality (p<0.0001), 20% lower progression (p=0.43), and 31% faster viral clearance (p=0.26).
Retrospective 863 Covid-19 patients in Burkina Faso, showing lower mortality, lower progression for outpatients, and faster viral clearance with HCQ/CQ treatment. Only the lower mortality was statistically significant. NCT04445441. https://c19p.org/rouamba
15. O. Mitjà, M. Corbacho-Monné, M. Ubals, A. Alemany, C. Suñer, C. Tebé, A. Tobias, J. Peñafiel, E. Ballana, C. Pérez, P. Admella, N. Riera-Martí, P. Laporte, J. Mitjà, M. Clua, L. Bertran, M. Sarquella, S. Gavilán, J. Ara, J. Argimon, G. Cuatrecasas, P. Cañadas, A. Elizalde-Torrent, R. Fabregat, M. Farré, A. Forcada, G. Flores-Mateo, C. López, E. Muntada, N. Nadal, S. Narejos, A. Nieto, N. Prat, J. Puig, C. Quiñones, F. Ramírez-Viaplana, J. Reyes-Urueña, E. Riveira-Muñoz, L. Ruiz, S. Sanz, A. Sentís, A. Sierra, C. Velasco, R. Vivanco-Hidalgo, J. Zamora, J. Casabona, M. Vall-Mayans, C. González-Beiras, and B. Clotet, A Cluster-Randomized Trial of Hydroxychloroquine as Prevention of Covid-19 Transmission and Disease Jul 2020, NEJM, Volume 384, Issue 5, Page 417-427
2,497 patient HCQ prophylaxis RCT: 46% lower mortality (p=0.39), 17% lower hospitalization (p=0.71), and 32% fewer cases (p=0.27).
For positive symptomatic cases, a greater effect is seen for nursing home residents, RR=0.49 [0.21 – 1.17], vs. overall 0.89, possibly because the exposure events are identified faster in this context, versus home exposure where testing of the source may be more delayed. The trial is too small for significance. If the trend continued this result would be significant at p<0.05 after about 25% more patients were added. There are 2 groups in this study: PCR+ at baseline (n=314) and PCR- at baseline (n=2000), which should be separated as they are different populations (primary outcome rates 18.6% and 22.2% compared to 3.0% and 4.3%). PCR+ already have Covid-19, so PEP analysis should be for the 2,000 PCR-, showing symptomatic Covid-19 of 4.3% (control) and 3.0% (treatment), RR 0.7, p=0.154. The paper has different RR values here, stating that they are adjusted for contact-level variables. It is not clear how they are computed – the adjusted RR for the overall sample is 4% lower, for PCR+ it is 20% lower, but for PCR- it is 107% higher, even though PCR- represents 86% of the sample. Hopefully, supplementary data will provide a breakdown on cases in this PCR- @baseline sample by number of days since exposure, and also provide relevant hospitalization and death results. Enrollment was up to 7 days after exposure, median 4 days. Treatment delay is unclear. The exposure event timing is not detailed. It appears to be based on the date of a positive test for a contact, which is likely to be much later than the actual exposure time. 13.1% were already positive at baseline, which is consistent with the actual exposure time being significantly earlier. PCR testing has a very high false-negative rate in early stages (e.g., 100% on day 1, 67% on day 4, and 20% on day 8), hence it is likely that a much higher percentage were infected at an unknown time before enrollment. Medication administration is not detailed. Sensitivity and specificity of the tests is not provided. Given the delay in identifying index cases, PCR test delay, and PCR false negative rate at early stages, the treatment delay in general was very long and could be over 2 weeks. The RR for non-PCR positive at baseline is 0.74. Including the PCR-positive at baseline patients reduced this to 0.89. This is also consistent with earlier treatment being more effective. The paper does not mention zinc. Zinc deficiency in Spain has been reported at 83%; this may significantly reduce effectiveness. HCQ is a zinc ionophore which increases cellular uptake, facilitating significant intracellular concentrations of zinc, and zinc is known to inhibit SARS-CoV RNA-dependent RNA polymerase activity, and is widely thought to be important for effectiveness with SARS-CoV-2. This study focuses on the existence of symptoms or PCR-positive results; however severity of symptoms is more important. Research has shown HCQ concentrations can be much higher in the lung compared to plasma, which may help minimize the occurrence of severe cases and death. There is a treatment-delay response relationship consistent with an effective treatment, however the authors only provide 3 ranges and do not break down the earliest treatment delay times. The definition of Covid-19 symptoms is very broad – just the existence of a headache alone or muscle pain alone was considered Covid-19. There was an overall very low incidence of confirmed Covid-19 (138 cases across both arms). There were no serious adverse events that were adjudicated as being treatment related. Authors exclude those with symptoms in the previous two weeks; however, those with symptoms up to several months before may still test PCR-positive even though there may be no viable virus. There appears to be incorrect data. Table 2, secondary outcomes, control, hospital/vital records shows that 8 of 1042 is 9.7% (calculated to be 0.8%). Nasopharyngeal viral load analysis issues include test unreliability and temporo-spatial differences in viral shedding. Data from this study has been used to show that viral load is the primary factor in transmission. https://c19p.org/mitjapep
16. J. Beltran Gonzalez, M. González Gámez, E. Mendoza Enciso, R. Esparza Maldonado, D. Hernández Palacios, S. Dueñas Campos, I. Robles, M. Macías Guzmán, A. García Díaz, C. Gutiérrez Peña, L. Martinez Medina, V. Monroy Colin, and J. Arreola Guerra, Efficacy and Safety of Ivermectin and Hydroxychloroquine in Patients with Severe COVID-19: A Randomized Controlled Trial Feb 2021, Infectious Disease Reports, Volume 14, Issue 2, Page 160-168
LATE TREATMENT 70 patient HCQ late treatment RCT: 63% lower mortality (p=0.27) and 25% lower progression (p=0.57).
RCT late-stage severe condition (93% SOFA ≥ 2, 96% APACHE ≥ 8) high comorbidity hospitalized patients in Mexico with 33 HCQ and 37 control patients not finding significant differences. NCT04391127. https://c19p.org/beltrangonzalezh
17. D. Rathod, K. Kargirwar, M. Patel, V. Kumar, K. Shalia, P. Singhal, Risk Factors associated with COVID-19 Patients in India: A Single Center Retrospective Cohort Study May 2023, The J. the Association of Physicians of India
EARLY TREATMENT 565 patient HCQ early treatment study: 73% lower mortality (p=0.02).
Retrospective 565 Covid-19 patients in India, showing lower mortality with HCQ+AZ treatment. Most patients (66%) had mild disease at baseline. https://c19p.org/rathod2
18. E. Heras, P. Garibaldi, M. Boix, O. Valero, J. Castillo, Y. Curbelo, E. Gonzalez, O. Mendoza, M. Anglada, J. Miralles, P. Llull, R. Llovera, and J. Piqué, COVID-19 mortality risk factors in older people in a long-term care center Sep 2020, European Geriatric Medicine, Volume 12, Issue 3, Page 601-607
EARLY TREATMENT 100 patient HCQ early treatment study: 96% lower mortality (p=0.004).
Retrospective 100 COVID+ elderly nursing home patients, HCQ+AZ mortality 11.4% vs. control 61.9%, RR 0.18, p<0.001. Median age 85. https://c19p.org/heras
19. M. Bernabeu-Wittel, J. Ternero-Vega, M. Nieto-Martín, L. Moreno-Gaviño, C. Conde-Guzmán, J. Delgado-Cuesta, M. Rincón-Gómez, P. Díaz-Jiménez, L. Giménez-Miranda, J. Lomas-Cabezas, M. Muñoz-García, S. Calzón-Fernández, and M. Ollero-Baturone, Effectiveness of a On-Site Medicalization Program for Nursing Homes with COVID-19 Outbreaks Jul 2020, J. Gerontol. A Biol. Sci. Med. Sci., Volume 76, Issue 3, Page e19-e27
EARLY TREATMENT 272 patient HCQ early treatment study: 94% lower mortality (p=0.001).
Retrospective 272 nursing home residents showing significantly improved survival after establishing a treatment program including HCQ with or without lopinavir/ritonavir and with the addition of adjuvant and antimicrobial treatments depending on circumstances. HCQ (114 patients), HCQ+LPV/RTV (18 patients), and HCQ+AZ (7 patients). Dosage details are in the supplementary appendix. https://c19p.org/bernabeuwittel
20. R. Polo, X. García-Albéniz, C. Terán, M. Morales, D. Rial-Crestelo, M. Garcinuño, M. García del Toro, C. Hita, J. Gómez-Sirvent, L. Buzón, A. Díaz de Santiago, J. Pérez Arellano, J. Sanz, P. Bachiller, E. Martínez Alfaro, V. Díaz-Brito, M. Masiá, A. Hernández-Torres, J. Guerra, J. Santos, P. Arazo, L. Muñoz, J. Arribas, P. Martínez de Salazar, S. Moreno, M. Hernán, J. Del Amo, J. Del Amo, Rosa Polo, S. Moreno, J. Berenguer, E. Martínez, M. Hernán, P. Martínez de Salazar, X. García de Albéniz, M. Iradier, I. Jarrín, J. Zamora, A. Rivero, C. Menéndez, E. Conde, J. Montes, C. Terán, B. Flores, M. Elena Choque, J. Peñaranda, G. Gorena, M. Herrera, M. Farfán, D. Moya et al., Daily tenofovir disoproxil fumarate/emtricitabine and hydroxychloroquine for pre-exposure prophylaxis of COVID-19: a double-blind placebo controlled randomized trial in healthcare workers Aug 2022, Clinical Microbiology and Infection
435 patient HCQ prophylaxis RCT: 51% fewer symptomatic cases (p=0.79) and 27% fewer cases (p=0.31).
Early terminated healthcare worker prophylaxis RCT in Spain, showing lower risk of symptomatic cases with HCQ prophylaxis, without statistical significance due to the small number of events. https://c19p.org/polo
21. V. Dubée, P. Roy, B. Vielle, E. Parot-Schinkel, O. Blanchet, A. Darsonval, C. Lefeuvre, C. Abbara, S. Boucher, E. Devaud, O. Robineau, P. Rispal, T. Guimard, E. D’Anglejean, S. Diamantis, M. Custaud, I. Pellier, A. Mercat, A. Brangier, P. Codron, J. Lemée, V. Pichon, R. Dhersin, G. Urbanski, C. Lavigne, R. Courtois, H. Danielou, J. Lebreton, R. Vatan, N. Crochette, J. Lainé, L. Perez, S. Blanchi, H. Hitoto, L. Bernard, F. Maillot, S. Marchand Adam, J. Talarmin, E. Gaigneux, P. Motte-Vincent, M. Morrier, D. Merrien, Y. Bleher, M. Flori, A. Ducet-Boiffard, O. Colin, R. Février, P. Thill, M. Tetart, F. Demaeght et al., Hydroxychloroquine in mild-to-moderate COVID-19: a placebo-controlled double blind trial Oct 2020, Clinical Microbiology and Infection, Volume 27, Issue 8, Page 1124-1130
LATE TREATMENT 247 patient HCQ late treatment RCT: 46% lower mortality (p=0.21) and 26% lower combined mortality/intubation (p=0.48).
Small early terminated late stage (60% on oxygen) RCT in France showing 46% lower mortality. Mortality at 28 days relative risk RR 0.54 [0.21-1.42] combined mortality/intubation at 28 days relative risk RR 0.74 [0.33-1.70]. If not stopped early and the same trend continued, statistical significance would be reached on 28-day mortality after ~550 patients (1,300 patients were planned). Mortality results are not provided for subgroups. For the subgroups receiving AZ: No safety concerns were identified. This study has been presented as negative; however the results do not support that conclusion. https://c19p.org/dubee
22. R. Amaravadi, L. Giles, M. Carberry, M. Hyman, I. Frank, S. Nasta, J. Walsh, E. Wileyto, P. Gimotty, M. Milone, E. Teng, N. Vyas, S. Balian, J. Kolansky, N. Abdulhay, S. McGovern, S. Gamblin, O. Doran, P. Callahan, and B. Abella, Hydroxychloroquine for SARS-CoV-2 positive patients quarantined at home: The first interim analysis of a remotely conducted randomized clinical trial Feb 2021, medRxiv
EARLY TREATMENT 29 patient HCQ early treatment RCT: 60% improved recovery (p=0.13).
Tiny early-terminated 34 patient RCT for outpatient treatment showing faster recovery with treatment (not statistically significant). All patients recovered (3 control patients recovered after crossover to the treatment arm) – as per protocol mid-recovery results have priority. There was no mortality and only one hospitalization on day 0 before treatment. There were no severe adverse events. https://c19p.org/amaravadi
23. S. Azhar, J. Akram, W. Latif, N. Cano Ibanez, S. Mumtaz, A. Rafi, U. Aftab, S. Iqtadar, M. Shahzad, F. Syed, B. Zafar, N. Fatima, S. Saadat Afridi, S. Javed Akram, M. Afzal Chaudhary, F. Sadiq, S. Goraya, M. Haneef, V. Ashraf, S. Ashraf, H. Akrma, and T. Khaliq, Effectiveness of early pharmaceutical interventions in symptomatic COVID-19 patients: A randomized clinical trial Mar 2024, Pakistan J. Medical Sciences, Volume 40, Issue 5
EARLY TREATMENT 471 patient HCQ early treatment RCT: 71% lower mortality (p=0.03), 4% greater improvement (p=0.64), and 10% improved viral clearance (p=0.52).
RCT 471 mild Covid-19 patients in Pakistan showing no significant differences in clinical improvement and viral clearance between HCQ, azithromycin, oseltamivir, and combinations. Mortality was significantly lower in HCQ vs. non-HCQ arms. The best results for viral clearance and clinical improvement were seen with the combination of all treatments. There was no control group. No serious adverse events were reported. All patients had mild Covid-19 and the paper indicates early treatment, however time from onset is not reported and minimal baseline information is provided. https://c19p.org/azhar
24. R. Derwand, M. Scholz, and V. Zelenko, COVID-19 Outpatients – Early Risk-Stratified Treatment with Zinc Plus Low Dose Hydroxychloroquine and Azithromycin: A Retrospective Case Series Study Jul 2020, Int. J. Antimicrobial Agents, Volume 56, Issue 6, Page 106214
EARLY TREATMENT 518 patient HCQ early treatment study: 79% lower mortality (p=0.12) and 82% lower hospitalization (p=0.001).
79% lower mortality and 82% lower hospitalization with early HCQ+AZ+Z. No cardiac side effects. Retrospective 518 patients (141 treated, 377 control). https://c19p.org/derwand
25. V. Guérin, P. Lévy, J. Thomas, T. Lardenois, P. Lacrosse, E. Sarrazin, N. Andreis, and M. Wonner, Azithromycin and Hydroxychloroquine Accelerate Recovery of Outpatients with Mild/Moderate COVID-19 May 2020, Asian J. Medicine and Health, July 15, 2020, Page 45-55
EARLY TREATMENT 88 patient HCQ early treatment study: 65% faster recovery (p=0.0001).
Mean clinical recovery time reduced from 26 days (standard-of-care treatment) to 9 days, p<0.0001 (HCQ+AZ) or 13 days, p<0.0001 (AZ). No cardiac toxicity. Small retrospective study of 88 patients with case control analysis with matched patients. https://c19p.org/guerin
26. T. Tarjoman, M. Valizadeh, P. Shojaei, B. Farhoodi, M. Zangeneh, M. Najafi, S. Jamaldini, M. Mesgarian, Z. Hanifezadeh, F. َAbdollahi, H. Massumi Naini, M. Alijani, H. Ziaee, and A. Chouhdari, The prophylactic effect of hydroxychloroquine on the severity of COVID-19 infection in an asymptomatic population: A randomized clinical trial Jan 2024, Social Determinants of Health, Volume 10, Issue Vol. 10 (2024): Continuous Issue
1,000 patient HCQ prophylaxis RCT: 80% lower hospitalization (p=0.25) and 43% fewer cases (p=0.005).
RCT of 1,000 people showing lower risk of Covid-19 infection with HCQ prophylaxis. There was no significant difference in side effects or adherence, no severe side effects, and blinding was well maintained. There are now PrEP RCTs, showing significant efficacy for Covid-19 cases. https://c19p.org/chouhdari
27. C. Yilgwan, A. Onu, J. Ofoli, L. Dakum, N. Shehu, D. Ogoina, I. Okoli, D. Osisanwo, V. Okafor, A. Olayinka, I. Mamadu, A. Adebiyi, Clinical profile and Predictors of Outcomes of Hospitalized Patients with Laboratory-Confirmed Severe Acute Respiratory Syndrome Coronavirus 2 in Nigeria: A Retrospective Analysis of 13 High Burden States in Nigeria May 2023, Nigerian Medical J.
LATE TREATMENT 3,462 patient HCQ late treatment study: 93% lower mortality (p<0.0001).
Retrospective 3,462 hospitalized Covid-19 patients across 13 states in Nigeria, showing lower mortality with HCQ. Authors note that the improved results compared with many other late stage studies may be related to the dose and experience of the physicians – in other studies beneficial effects may be offset by the side effects of high cumulative doses in late-stage patients. Authors also note the worse results with a combination of CQ/HCQ and AZ may be related to the side effects becoming more significant for late stage patients. https://c19p.org/yilgwan
28. B. Obrișcă, A. Vornicu, R. Jurubiță, V. Mocanu, G. Dimofte, A. Andronesi, B. Sorohan, C. Achim, G. Micu, R. Bobeică, C. Dina, and G. Ismail, Characteristics of SARS-CoV-2 Infection in an Actively Monitored Cohort of Patients with Lupus Nephritis Sep 2022, Biomedicines, Volume 10, Issue 10, Page 2423
95 patient HCQ prophylaxis study: 87% fewer cases (p=0.01).
Prospective analysis of 95 Lupus Nephritis patients in Romania, showing lower risk of Covid-19 with HCQ use. https://c19p.org/obrisca
29. C. Loucera, R. Carmona, M. Esteban-Medina, G. Bostelmann, D. Muñoyerro-Muñiz, R. Villegas, M. Peña-Chilet, and J. Dopazo, Real-world evidence with a retrospective cohort of 15,968 COVID-19 hospitalized patients suggests 21 new effective treatments Aug 2022, Virology J., Volume 20, Issue 1
15,968 patient HCQ prophylaxis study: 69% lower mortality (p=0.0002).
Retrospective 15,968 Covid-19 hospitalized patients in Spain, showing lower mortality with existing use of several medications including metformin, HCQ, azithromycin, aspirin, vitamin D, vitamin C, and budesonide. Since only hospitalized patients are included, results do not reflect different probabilities of hospitalization across treatments. https://c19p.org/loucera3h
30. D. Badyal, S. Chandy, P. Chugh, A. Faruqui, Y. Gupta, A. Hazra, S. Kamat, V. Kamboj, R. Kaul, N. Kshirsagar, S. Maulik, B. Medhi, G. Menon, J. Ranjalkar, V. Rao, Y. Shetty, R. Tripathi, D. Xavier, Hydroxychloroquine for SARS CoV2 Prophylaxis in Healthcare Workers – A Multicentric Cohort Study Assessing Effectiveness and Safety Jun 2021, J. the Association of Physicians of India, June 2021
2,090 patient HCQ prophylaxis study: 60% fewer cases (p<0.0001).
Prophylaxis study with 12,089 Indian healthcare workers, showing lower risk of Covid-19 cases with treatment, and increasingly lower risk for longer durations of HCQ prophylaxis. The appendices were not available. https://c19p.org/badyal
31. J. Rojas-Serrano, A. Portillo-Vásquez, I. Thirion-Romero, J. Vázquez-Pérez, F. Mejía-Nepomuceno, A. Ramírez-Venegas, K. Pérez-Kawabe, and R. Pérez-Padilla, Hydroxychloroquine for prophylaxis of COVID-19 in health workers: A randomized clinical trial May 2021, PLOS ONE, Volume 17, Issue 2, Page e0261980
127 patient HCQ prophylaxis RCT: 82% fewer symptomatic cases (p=0.12).
Early terminated HCQ PrEP RCT with 62 HCQ and 65 placebo patients, showing 82% lower cases with treatment, p = 0.12. If the trial is continued and the same event rate is observed, statistical significance would have been reached after adding about 16 patients per arm. https://c19p.org/rojasserrano
32. E. Corradini, P. Ventura, W. Ageno, C. Cogliati, M. Muiesan, D. Girelli, M. Pirisi, A. Gasbarrini, P. Angeli, P. Querini, E. Bosi, M. Tresoldi, R. Vettor, M. Cattaneo, F. Piscaglia, A. Brucato, S. Perlini, P. Martelletti, R. Pontremoli, M. Porta, P. Minuz, O. Olivieri, G. Sesti, G. Biolo, D. Rizzoni, G. Serviddio, F. Cipollone, D. Grassi, R. Manfredini, G. Moreo, A. Pietrangelo, E. Tombolini, T. Teatini, E. Crisafulli, P. Sainaghi, L. Zileri Dal Verme, S. Piano, R. De Lorenzo, G. Arcidiacono, M. Podda, L. Muratori, C. Gabiati, F. Salinaro, M. Luciani, C. Barnini, S. Morra di Cella, A. Dalbeni, S. Friso, M. Luciani, F. Mearelli et al., Clinical factors associated with death in 3044 COVID-19 patients managed in internal medicine wards in Italy: results from the SIMI-COVID-19 study of the Italian Society of Internal Medicine (SIMI) Apr 2021, Internal and Emergency Medicine, Volume 16, Issue 4, Page 1005-1015
LATE TREATMENT 1,713 patient HCQ late treatment study: 70% lower mortality (p<0.0001).
Retrospective 3,044 hospitalized Covid-19 patients in Italy, showing HCQ significantly associated with survival in light, mild, and moderate cases in multivariable analysis, but not in severe cases. https://c19p.org/corradini
33. B. Cangiano, L. Fatti, L. Danesi, G. Gazzano, M. Croci, G. Vitale, L. Gilardini, S. Bonadonna, I. Chiodini, C. Caparello, A. Conti, L. Persani, M. Stramba-Badiale, and M. Bonomi, Mortality in an Italian nursing home during COVID-19 pandemic: correlation with gender, age, ADL, vitamin D supplementation, and limitations of the diagnostic tests Dec 2020, Aging, Volume 12, Issue 24, Page 24522-24534
LATE TREATMENT 98 patient HCQ late treatment study: 73% lower mortality (p=0.03).
Analysis of 98 PCR+ nursing home residents in Italy, mean age 90, showing HCQ mortality RR 0.27, p = 0.03. Subject to confounding by contraindication. The paper provides the p value for regression but not the effect size. https://c19p.org/cangiano
34. E. Sheshah, S. Sabico, R. Albakr, A. Sultan, K. Alghamdi, K. Al Madani, H. Alotair, and N. Al-Daghri, Prevalence of Diabetes, Management and Outcomes among Covid-19 Adult Patients Admitted in a Specialized Tertiary Hospital in Riyadh, Saudi Arabia Nov 2020, Diabetes Research and Clinical Practice, Volume 172, Page 108538
LATE TREATMENT 300 patient HCQ late treatment study: 80% lower mortality (p=0.001).
Retrospective 300 hospitalized patients in Saudi Arabia showing HCQ adjusted odds ratio adjusted odds ratio 0.12, p < 0.001. https://c19p.org/sheshah
35. I. Simova, T. Vekov, J. Krasnaliev, V. Kornovski, and P. Bozhinov, Hydroxychloroquine for prophylaxis and treatment of COVID-19 in health care workers Nov 2020, New Microbes and New Infections, Volume 38, Page 100813
204 patient HCQ prophylaxis study: 93% fewer cases (p=0.01).
100% reduction in cases with HCQ+zinc post-exposure prophylaxis. Brief report for healthcare workers in Bulgaria. 0 cases with treatment vs. 3 for control. 156 treatment patients and 48 control patients. No serious adverse events. This paper reports on both PEP and early treatment, we have separated the two studies. https://c19p.org/simovapep
36. V. Hande, S. Mathai, and V. Behera, Hydroxychloroquine as pre-exposure prophylaxis against COVID-19 in health-care workers: A single-center experience Nov 2020, J. Marine Medical Society, Volume 0, Issue 0, Page 0
604 patient HCQ prophylaxis study: 90% fewer cases (p<0.0001).
90% reduction in cases with HCQ pre-exposure prophylaxis. Retrospective 604 healthcare workers. https://c19p.org/mathai
37. J. Nogueira López, C. Grasa Lozano, C. Ots Ruiz, L. Alonso García, I. Falces-Romero, C. Calvo, and M. García-López Hortelano, Telemedicine follow-ups for COVID-19: experience in a tertiary hospital Nov 2020, Annals of Pediatrics, Volume 95, Issue 5, Page 336-344
LATE TREATMENT 72 patient HCQ late treatment study: 64% lower progression (p=0.02).
Retrospective 72 pediatric patients showing HCQ associated with a shorter duration of fever (p=0.023), less progression (p=0.016), and fewer return visits to the ER (p=0.017). https://c19p.org/lopez2
38. M. Lauriola, A. Pani, G. Ippoliti, A. Mortara, S. Milighetti, M. Mazen, G. Perseghin, D. Pastori, P. Grosso, and F. Scaglione, Effect of combination therapy of hydroxychloroquine and azithromycin on mortality in COVID-19 patients Sep 2020, Clinical and Translational Science, Volume 13, Issue 6, Page 1071-1076
LATE TREATMENT 360 patient HCQ late treatment study: 74% lower mortality (p=0.001).
Retrospective 377 patients, 73% reduction in mortality with HCQ+AZ, adjusted hazard ratio 0.27 [0.17-0.41]. Mean age 71.8. No serious adverse events. Subject to incomplete adjustment for confounders. https://c19p.org/lauriola
39. C. Ferri, D. Giuggioli, V. Raimondo, M. L’Andolina, A. Tavoni, R. Cecchetti, S. Guiducci, F. Ursini, M. Caminiti, G. Varcasia, P. Gigliotti, R. Pellegrini, D. Olivo, M. Colaci, G. Murdaca, R. Brittelli, G. Mariano, A. Spinella, S. Bellando-Randone, V. Aiello, S. Bilia, D. Giannini, T. Ferrari, R. Caminiti, V. Brusi, R. Meliconi, P. Fallahi, and A. Antonelli, COVID-19 and rheumatic autoimmune systemic diseases: report of a large Italian patients series Aug 2020, Clinical Rheumatology, Volume 39, Issue 11, Page 3195-3204
1,641 patient HCQ prophylaxis study: 63% fewer cases (p=0.02).
Analysis of 1641 systemic autoimmune disease patients showing csDMARD (HCQ etc.) RR 0.37, p=0.015. csDMARDs include HCQ, CQ, and several other drugs, so the effect of HCQ/CQ alone could be higher. This study also confirms that the risk of Covid-19 for systemic autoimmune disease patients is much higher overall, OR 4.42, p<0.001 (this is the observed real-world risk which takes into account factors such as these patients potentially being more careful to avoid exposure). (results are for “definite + highly suspected” cases and the main result is presented in the paper as the OR for not taking csDMARDs, c19early converted this to RR. https://c19p.org/ferri
40. A. Dubernet, K. Larsen, L. Masse, J. Allyn, E. Foch, L. Bruneau, A. Maillot, M. Lagrange-Xelot, V. Thomas, M. Jaffar-Bandjee, L. Gauzere, L. Raffray, K. Borsu, S. Dibernardo, S. Renaud, M. André, D. Moreau, J. Jabot, N. Coolen-Allou, and N. Allou, A comprehensive strategy for the early treatment of COVID-19 with azithromycin/hydroxychloroquine and/or corticosteroids: results of a retrospective observational study in the French overseas department of Reunion Island Aug 2020, J. Global Antimicrobial Resistance, Volume 23, Page 1-3
LATE TREATMENT 36 patient HCQ late treatment study: 88% lower ICU admission (p=0.008).
Retrospective analysis of 36 hospitalized patients showing HCQ/AZ associated with lower ICU admission, p=0.008. Median age 66, no mortality. Confounding by indication; however, it was patients with hypoxemic pneumonia that were treated with HCQ/AZ. Patients were not treated with HCQ/AZ if they didn’t need oxygen therapy. Even at that late stage, it showed efficacy. https://c19p.org/dubernet
41. M. Soneja, H. Kadnur, A. Aggarwal, K. Singh, A. Mittal, N. Nischal, P. Tirlangi, A. Khan, D. Desai, A. Gupta, A. Kumar, P. Jorwal, A. Biswas, R. Pandey, N. Wig, and R. Guleria, Hydroxychloroquine pre-exposure prophylaxis for COVID-19 among healthcare workers: Initial experience from India Jul 2020, J. Family Medicine and Primary Care, Volume 11, Issue 3, Page 1140
358 patient HCQ prophylaxis study: 62% fewer cases (p=0.01).
Prophylaxis study with 334 low-risk healthcare workers in India, showing significantly lower risk of cases with treatment. Symptomatic patients received PCR results, but only some asymptomatic patients did, so there may have been additional asymptomatic cases. There were no severe adverse events. https://c19p.org/kadnur
42. J. Zhong, G. Shen, H. Yang, A. Huang, X. Chen, L. Dong, B. Wu, A. Zhang, L. Su, X. Hou, S. Song, H. Li, W. Zhou, T. Zhou, Q. Huang, A. Chu, Z. Braunstein, X. Rao, C. Ye, and L. Dong, COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study Jul 2020, Lancet Rheumatology, Volume 2, Issue 9, Page e557-e564
43 patient HCQ prophylaxis study: 91% fewer cases (p=0.04).
Rheumatic disease patients on HCQ had a lower risk of Covid-19 than those on other disease-modifying anti-rheumatic drugs, OR 0.09 (0.01–0.94), p=0.044 after adjusting for age, sex, smoking, systemic lupus erythematosus, infection in other family members, and comorbidities. 43 patients with rheumatic disease and Covid-19 exposure. https://c19p.org/zhong
43. J. Rogado, C. Pangua, G. Serrano-Montero, B. Obispo, A. Marino, M. Pérez-Pérez, A. López-Alfonso, P. Gullón, and M. Lara, Covid-19 and lung cancer: A greater fatality rate? May 2020, Lung Cancer, Volume 146, Page 19-22
LATE TREATMENT 17 patient HCQ late treatment study: 92% lower mortality (p=0.02).
Retrospective 17 hospitalized lung cancer patients showing lower mortality with HCQ+AZ treatment. https://c19p.org/rogado
44. S. Panda, P. Chatterjee, T. Anand, K. Singh, R. Rasaily, R. Singh, S. Das, H. Singh, I. Praharaj, R. Gangakhedkar, and B. Bhargava, Healthcare workers & SARS-CoV-2 infection in India: A case-control investigation in the time of COVID-19 May 2020, Indian J. Med. Res., June 20, 2020, Volume 151, Issue 5, Page 459
455 patient HCQ prophylaxis study: 67% fewer cases (p=0.001).
4+ doses of HCQ associated with a significant decline in the odds of getting infected, dose-response relationship exists. https://c19p.org/chatterjee
45. M. Huang, M. Li, F. Xiao, P. Pang, J. Liang, T. Tang, S. Liu, B. Chen, J. Shu, Y. You, Y. Li, M. Tang, J. Zhou, G. Jiang, J. Xiang, W. Hong, S. He, Z. Wang, J. Feng, C. Lin, Y. Ye, Z. Wu, Y. Li, B. Zhong, R. Sun, Z. Hong, J. Liu, H. Chen, X. Wang, Z. Li, D. Pei, L. Tian, J. Xia, S. Jiang, N. Zhong, and H. Shan, Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19 May 2020, National Science Review, nwaa113, Volume 7, Issue 9, Page 1428-1436
LATE TREATMENT 373 patient HCQ late treatment study: 67% faster viral clearance (p=0.0001).
197 CQ patients, 176 control. Mean time to undetectable viral RNA and duration of fever significantly reduced. No serious adverse events. https://c19p.org/huangnsr
46. B. Yu, C. Li, P. Chen, N. Zhou, L. Wang, J. Li, H. Jiang, and D. Wang, Low Dose of Hydroxychloroquine Reduces Fatality of Critically Ill Patients With COVID-19 May 2020, Science China Life Sciences, 2020 May 15, 1-7, Volume 63, Issue 10, Page 1515-1521
LATE TREATMENT 550 patient HCQ late treatment study: 60% lower mortality (p=0.002).
Retrospective, 550 critically ill patients. 19% fatality for HCQ versus 47% for non-HCQ, RR 0.395, p=0.002. The levels of inflammatory cytokine IL-6 were significantly reduced from 22.2 pg/mL to 5.2 pg/mL (p<0.05) at the end of the treatment in the HCQ group but there was no change in the control group. https://c19p.org/yu
47. A. Pate, A. Shankarkumar, S. Shinde, M. Pruthi, H. Patil, and M. Madkaikar, Sero-survey for health-care workers provides corroborative evidence for the effectiveness of Hydroxychloroquine prophylaxis against COVID-19 infection Sep 2020, ResearchGate
500 patient HCQ prophylaxis study: 82% lower hospitalization (p=0.01) and 42% fewer cases (p=0.05).
ICMR seroprevalence survey of 500 healthcare workers in India, 279 taking HCQ prophylaxis, showing a significantly lower risk with treatment, and lower severity. https://c19p.org/yadav3
48. M. Mokhtari, M. Mohraz, M. Gouya, H. Namdari Tabar, J. Tabrizi, K. Tayeri, S. Aghamohamadi, Z. Rajabpoor, M. Karami, A. Raeisi, H. Rahmani, and H. Khalili, Clinical outcomes of patients with mild COVID-19 following treatment with hydroxychloroquine in an outpatient setting Apr 2021, Int. Immunopharmacology, Volume 96, Page 107636
EARLY TREATMENT 28,759 patient HCQ early treatment study: 70% lower mortality (p<0.0001) and 35% lower hospitalization (p<0.0001).
Retrospective 28,759 adult outpatients with mild Covid-19 in Iran, 7,295 treated with HCQ, showing significantly lower hospitalization and mortality with treatment. https://c19p.org/mokhtari
49. M. AlQahtani, N. Kumar, D. Aljawder, A. Abdulrahman, M. Mohamed, F. Alnashaba, M. Fayyad, F. Alshaikh, F. Alsahaf, S. Saeed, A. Almahroos, Z. Abdulrahim, S. Otoom, and S. Atkin, Randomized controlled trial of favipiravir, hydroxychloroquine, and standard care in patients with mild/moderate COVID-19 disease Mar 2022, Scientific Reports, Volume 12, Issue 1
LATE TREATMENT 103 patient HCQ late treatment RCT: 4% improved recovery (p=0.94) and 47% improved viral clearance (p=0.13).
RCT with 54 favipiravir, 51 HCQ, and 52 standard-of-care hospitalized patients in Bahrain, showing no significant differences. Viral clearance improved with both treatments, but did not reach statistical significance with the small sample size. https://c19p.org/alqahtani2
50. A. Ip, J. Ahn, Y. Zhou, A. Goy, E. Hansen, A. Pecora, B. Sinclaire, U. Bednarz, M. Marafelias, I. Sawczuk, J. Underwood, D. Walker, R. Prasad, R. Sweeney, M. Ponce, S. La Capra, F. Cunningham, A. Calise, B. Pulver, D. Ruocco, G. Mojares, M. Eagan, K. Ziontz, P. Mastrokyriakos, and S. Goldberg, Hydroxychloroquine in the treatment of outpatients with mildly symptomatic COVID-19: A multi-center observational study Aug 2020, BMC Infectious Diseases, Volume 21, Issue 1
EARLY TREATMENT 1,067 patient HCQ early treatment study: 55% lower mortality (p=0.43) and 37% lower hospitalization (p=0.04).
Retrospective 1,274 outpatients, 47% reduction in hospitalization with HCQ with propensity matching, HCQ OR 0.53 [0.29-0.95]. Sensitivity analyses revealed similar associations. Adverse events were not increased (2% QTc prolongation events, 0% arrhythmias). https://c19p.org/ip
51. F. Cadegiani, A. Goren, C. Wambier, and J. McCoy, Early COVID-19 Therapy with azithromycin plus nitazoxanide, ivermectin or hydroxychloroquine in Outpatient Settings Significantly Improved COVID-19 outcomes compared to Known outcomes in untreated patients Nov 2020, New Microbes and New Infections, Volume 43, Page 100915
296 patient HCQ early treatment study: 81% lower mortality (p=0.21), 95% lower ventilation (p=0.0008), and 98% lower hospitalization (p<0.0001).
Comparison of HCQ, nitazoxanide, and ivermectin showing similar effectiveness for overall clinical outcomes in Covid-19 when used before seven days of symptoms, and overwhelmingly superior compared to the untreated Covid-19 population, even for those outcomes not influenced by placebo effect, at least when combined with azithromycin, and vitamin C, D and zinc in the majority of the cases. 585 patients with mean treatment delay 2.9 days. There was no hospitalization, mechanical ventilation, or mortality with treatment. Control group 1 was a retrospectively obtained group of untreated patients of the same population. https://c19p.org/cadegiani
52. D. Dhibar, N. Arora, D. Chaudhary, A. Prakash, B. Medhi, N. Singla, R. Mohindra, V. Suri, A. Bhalla, N. Sharma, M. Singh, P. Lakshmi, K. Goyal, and A. Ghosh, The ‘myth of Hydroxychloroquine (HCQ) as post-exposure prophylaxis (PEP) for the prevention of COVID-19’ is far from reality Jan 2023, Scientific Reports, Volume 13, Issue 1
1,168 patient HCQ prophylaxis RCT: 27% fewer symptomatic cases (p=0.32) and 21% fewer cases (p=0.21).
Low-dose low-risk patient HCQ PEP RCT, showing lower symptomatic cases with treatment, without statistical significance. There were no moderate or severe cases. HCQ 800mg on day one followed by 400mg once weekly for 3 weeks. https://c19p.org/dhibar2
53. T. Ly, D. Zanini, V. Laforge, S. Arlotto, S. Gentile, H. Mendizabal, M. Finaud, D. Morel, O. Quenette, P. Malfuson-Clot-Faybesse, A. Midejean, P. Le-Dinh, G. Daher, B. Labarriere, A. Morel-Roux, A. Coquet, P. Augier, P. Parola, E. Chabriere, D. Raoult, and P. Gautret, Pattern of SARS-CoV-2 infection among dependant elderly residents living in retirement homes in Marseille, France, March-June 2020 Aug 2020, Int. J. Antimicrobial Agents, Volume 56, Issue 6, Page 106219
EARLY TREATMENT 226 patient HCQ early treatment study: 56% lower mortality (p=0.02).
Retrospective analysis of retirement homes, HCQ+AZ >= 3 days mortality OR 0.37, p=0.02. 1,690 elderly residents (mean age 83), 226 infected residents, 116 treated with HCQ+AZ >= 3 days. Detection via mass screening also showed significant improvements (16.9% vs. 40.6%, OR 0.20, p=0.001), suggesting that earlier detection and treatment is more successful. https://c19p.org/ly
54. C. Skipper, K. Pastick, N. Engen, A. Bangdiwala, M. Abassi, S. Lofgren, D. Williams, E. Okafor, M. Pullen, M. Nicol, A. Nascene, K. Hullsiek, M. Cheng, D. Luke, S. Lother, L. MacKenzie, G. Drobot, L. Kelly, I. Schwartz, R. Zarychanski, E. McDonald, T. Lee, R. Rajasingham, and D. Boulware, Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19: A Randomized Trial Jul 2020, Annals of Internal Medicine, Volume 173, Issue 8, Page 623-631
465 patient HCQ “early treatment” RCT: 37% lower combined mortality/hospitalization (p=0.58), 49% lower hospitalization (p=0.38), and 20% improved recovery (p=0.21).
No details on treatment delay. An author reports that treatment initiation time was not recorded. Conflicting estimates are provided in a comment of the article and independent analysis, with reports indicating missing data in the dataset. Also see (companion PEP trial), and Pullen et al., which shows shipping delay for these trials of 19 – 68 hours. Only one-third of participants completed enrollment weekdays between 8:00am and 4:00pm, with 44% outside of these hours during the week, and 22% during the weekend. With enrollment up to 4 days after symptom onset, this implies delivery 19 – 164 hours after onset (19 hours would require instantaneous enrollment). ~70 to 140 hours (inc. shipping) delayed outpatient treatment with HCQ showing lower hospitalization/death and faster recovery, but not reaching statistical significance. There was one hospitalized control death and one non-hospitalized HCQ death. It is unclear why there was a non-hospitalized death; external factors such as lack of standard care may be involved. Excluding that case results in one control death and zero HCQ deaths. Details for the hospitalizations and deaths such as medication adherence and treatment delay would have been informative but are not provided. The paper states the endpoint was changed to symptom severity because they would have required 6,000 participants. However, if the same event rates continued, they would hit 95% significance on the reduction in hospitalization after adding less than 500 patients per arm. Treatment is relatively late, ~70 to 140 hours after symptoms, including the shipping delay. The paper does not mention the shipping delay but partial details are provided in the study protocol. They are not clear but suggest no shipping on the weekends and a possible 12pm cutoff for same day dispensing and mailing. Temperature violations occurring while mailing prescription not considered. Assuming that enrollments were evenly distributed between 6am and 12am each day, we get an average of approximately 46 hours shipping delay. Research shows the treatment used in the control arm (folic acid in the USA which was most patients) may have significant efficacy for Covid-19 [Deschasaux-Tanguy, Farag], so the true effectiveness of HCQ may be higher than observed. Also see this. Note that folic acid is predicted to bind to multiple SARS-CoV-2 proteins, folic acid levels are lower in Covid-19 patients with severe disease, folic acid supplementation may help with Covid-19 associated hypertension and hyperhomocysteinemia, and differences in a folic acid-related enzyme could impact Covid-19 geographical severity variation. The paper compares 0 – 36 hour delayed treatment with oseltamivir (used for influenza) and ~70 to 140 hour delayed treatment with HCQ (Covid-19), noting that oseltamivir seemed more effective. However, a more comparable study is McLean (2015) who showed that 48 – 119 hour delayed treatment with oseltamivir has no effect. This suggests that HCQ is more effective than oseltamivir, and that HCQ may still have significant effect for some amount of delay beyond the delay where oseltamivir is effective. 6 people were included that enrolled with >4d symptoms, even though they didn’t match the study inclusion criteria. This reduces observed effectiveness. The paper says 56% (236) were enrolled within 1 day of symptoms, but results show only 40% for “<1d”… 56% is possible for <48hrs, clarification is needed. Patients in this study are relatively young and most of them recover without assistance. This reduces the room for a treatment to make improvements. The maximum improvement of an effective treatment would be expected before all patients approach recovery. Authors focus on the end result where most have recovered, but it is more informative to examine the curve and the point of maximum effectiveness. Authors did not collect data for every day but they do have interim results for days 3, 5, 10. The results are consistent with an effective treatment and show a statistically significant improvement, p = 0.05, at day 10 (other unreported days might show increased effectiveness). Results also show a larger treatment effect for those >50, not statistically significant due to the small sample, but noted as Covid-19 risk dramatically increases with age. The effect may be more visible here because younger patients may on average have more mild cases with less room for improvement. In general, patients in this study have relatively mild symptoms on average, limiting the chance to observe improvement. The study relies on Internet surveys. Known fake surveys were submitted to the similar PEP trial and there could be an unknown number of undetected fake surveys in both trials. RCT of 423 patients with Internet surveys. Analysis of primarily low-risk patients; authors note the results are not generalizable to the Covid high-risk population. https://c19p.org/skipper
55. Smith et al., Evaluating the Efficacy of Hydroxychloroquine and Azithromycin to Prevent Hospitalization or Death in Persons With COVID-19 Jul 2020, NCT04358068
EARLY TREATMENT 16 patient HCQ early treatment RCT: 64% lower hospitalization (p=1) and 10% slower recovery.
Early terminated NIAID RCT for HCQ. Patients >60 were only in the HCQ arm. 57% of patients were high-risk in the HCQ arm vs. 22% for control. Treatment started up to 20 days after symptoms. https://c19p.org/smith2
56. M. Kim, S. Jang, Y. Park, B. Kim, T. Hwang, S. Kang, W. Kim, P. Kyu, H. Park, W. Yang, J. Jang, and M. An, Treatment Response to Hydroxychloroquine, Lopinavir/Ritonavir, and Antibiotics for Moderate COVID 19: A First Report on the Pharmacological Outcomes from South Korea May 2020, medRxiv
LATE TREATMENT 97 patient HCQ late treatment study: 51% shorter hospitalization (p=0.01) and 56% faster viral clearance (p=0.005).
Retrospective of 97 moderate cases. Time to viral clearance significantly shorter for HCQ+antibiotic. Preprint withdrawn pending peer review. https://c19p.org/kim
57. Novartis et al., Hydroxychloroquine Monotherapy and in Combination With Azithromycin in Patients With Moderate and Severe COVID-19 Disease Jul 2020, Novartis, NCT04358081
LATE TREATMENT 12 patient HCQ late treatment RCT: 71% higher hospital discharge (p=0.42), 71% greater improvement (p=0.42), and 79% worse viral clearance (p=0.56).
Early terminated RCT with only 20 patients. https://c19p.org/novartis
58. I. Núñez-Gil, L. Ayerbe, C. Fernandez-Pérez, V. Estrada, C. Eid, R. Arroyo-Espliguero, R. Romero, V. Becerra-Muñoz, A. Uribarri, G. Feltes, D. Trabattoni, M. Molina, M. Aguado, M. Pepe, E. Cerrato, J. Huang, T. Astrua, E. Alfonso, A. Castro-Mejía, S. Raposeiras-Roubin, L. Buzón, C. Paeres, A. Mulet, N. Lal-Trehan, E. Garcia-Vazquez, O. Fabregat-Andres, I. Akin, F. D´Ascenzo, P. Gomez-Rosado, F. Ugo, A. Fernández-Ortiz, and C. Macaya, Hydroxychloroquine and Mortality in SARS-Cov-2 Infection; The HOPE- Covid-19 Registry. Sep 2022, Anti-Infective Agents, Volume 20
LATE TREATMENT 6,217 patient HCQ late treatment PSM study: 53% lower mortality (p<0.0001).
Propensity score matching retrospective study of 6,217 hospitalized patients in Spain, showing lower mortality with HCQ. The higher efficacy reported with obesity associated with high serum cholesterol levels is consistent with the greater HCQ efficacy predicted for patients with higher cholesterol. https://c19p.org/nunezgil2
59. M. Ugarte-Gil, G. Alarcón, Z. Izadi, A. Duarte-García, C. Reátegui-Sokolova, A. Clarke, L. Wise, G. Pons-Estel, M. Santos, S. Bernatsky, S. Ribeiro, S. Al Emadi, J. Sparks, T. Hsu, N. Patel, E. Gilbert, M. Valenzuela-Almada, A. Jönsen, G. Landolfi, M. Fredi, T. Goulenok, M. Devaux, X. Mariette, V. Queyrel, V. Romão, G. Sequeira, R. Hasseli, B. Hoyer, R. Voll, C. Specker, R. Baez, V. Castro-Coello, H. Maldonado Ficco, E. Reis Neto, G. Ferreira, O. Monticielo, E. Sirotich, J. Liew, J. Hausmann, P. Sufka, R. Grainger, S. Bhana, W. Costello, Z. Wallace, L. Jacobsohn, T. Taylor, C. Ja, A. Strangfeld, E. Mateus, K. Hyrich et al., Characteristics associated with poor COVID-19 outcomes in individuals with systemic lupus erythematosus: data from the COVID-19 Global Rheumatology Alliance Feb 2022, Annals of the Rheumatic Diseases, Page annrheumdis-2021-221636
895 patient HCQ prophylaxis study: 44% lower severe cases (p=0.007).
Retrospective 1,606 SLE patients showing lower risk of severe Covid-19 outcomes with HCQ/CQ use. https://c19p.org/ugartegil
60. J. Lora-Tamayo, G. Maestro, A. Lalueza, M. Rubio-Rivas, G. Villarreal Paul, F. Arnalich Fernández, J. Beato Pérez, J. Vargas Núñez, M. Llorente Barrio, and C. Lumbreras Bermejo, Early Lopinavir/ritonavir does not reduce mortality in COVID-19 patients: results of a large multicenter study Feb 2021, J. Infection, Volume 82, Issue 6, Page 276-316
LATE TREATMENT 8,553 patient HCQ late treatment study: 50% lower mortality (p<0.0001).
Lopinavir/ritonavir retrospective study also showing univariate results for HCQ, with significantly lower mortality. https://c19p.org/loratamayo
61. A. Di Castelnuovo, A. Gialluisi, A. Antinori, N. Berselli, L. Blandi, M. Bonaccio, R. Bruno, R. Cauda, S. Costanzo, G. Guaraldi, L. Menicanti, M. Mennuni, I. My, G. Parruti, G. Patti, S. Perlini, F. Santilli, C. Signorelli, G. Stefanini, A. Vergori, W. Ageno, A. Agodi, P. Agostoni, L. Aiello, S. Al Moghazi, R. Arboretti, F. Aucella, G. Barbieri, M. Barchitta, P. Bonfanti, F. Cacciatore, L. Caiano, F. Cannata, L. Carrozzi, A. Cascio, G. Castiglione, A. Ciccullo, A. Cingolani, F. Cipollone, C. Colomba, C. Colombo, A. Crisetti, F. Crosta, G. Danzi, D. D’Ardes, K. De Gaetano Donati, F. Di Gennaro, G. Di Tano, G. D’Offizi, F. Fusco et al., Disentangling the Association of Hydroxychloroquine Treatment with Mortality in Covid-19 Hospitalized Patients through Hierarchical Clustering Jan 2021, J. Healthcare Engineering, Volume 2021, Page 1-10
LATE TREATMENT 4,270 patient HCQ late treatment study: 40% lower mortality (p<0.0001).
Retrospective 4,396 hospitalized patients in Italy showing significantly lower mortality with HCQ treatment, and identifying greater efficacy for a subgroup of patients in clustering analysis. https://c19p.org/dicastelnuovo2
62. A. Strangfeld, M. Schäfer, M. Gianfrancesco, S. Lawson-Tovey, J. Liew, L. Ljung, E. Mateus, C. Richez, M. Santos, G. Schmajuk, C. Scirè, E. Sirotich, J. Sparks, P. Sufka, T. Thomas, L. Trupin, Z. Wallace, S. Al-Adely, J. Bachiller-Corral, S. Bhana, P. Cacoub, L. Carmona, R. Costello, W. Costello, L. Gossec, R. Grainger, E. Hachulla, R. Hasseli, J. Hausmann, K. Hyrich, Z. Izadi, L. Jacobsohn, P. Katz, L. Kearsley-Fleet, P. Robinson, J. Yazdany, and P. Machado, Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry Jan 2021, Annals of the Rheumatic Diseases, Volume 80, Issue 7, Page 930-942
1,165 patient HCQ prophylaxis study: 48% lower mortality (p<0.0001).
Retrospective 3,729 rheumatic disease patients showing lower risk of mortality with HCQ/CQ use (HCQ/CQ vs. no DMARD therapy). https://c19p.org/strangfeld
63. J. Signes-Costa, I. Núñez-Gil, J. Soriano, R. Arroyo-Espliguero, C. Eid, R. Romero, A. Uribarri, I. Fernández-Rozas, M. Aguado, V. Becerra-Muñoz, J. Huang, M. Pepe, E. Cerrato, S. Raposeiras, A. Gonzalez, F. Franco-Leon, L. Wang, E. Alfonso, F. Ugo, J. García-Prieto, G. Feltes, M. Abumayyaleh, C. Espejo-Paeres, J. Jativa, A. Masjuan, C. Macaya, J. Carbonell Asíns, and V. Estrada, Prevalence and 30-day mortality in hospitalized patients with COVID-19 and prior lung diseases Dec 2020, Archivos de Bronconeumología, Volume 57, Page 13-20
LATE TREATMENT 5,847 patient HCQ late treatment study: 47% lower mortality (p=0.0005).
47% lower mortality with HCQ/CQ. Retrospective 1,271 patients with lung disease in Canada, China, Cuba, Ecuador, Germany, Italy, and Spain, 83% treated with HCQ/CQ. Multivariable Cox regression HCQ/CQ mortality hazard ratio HR 0.53, p < 0.001. https://c19p.org/signescosta
64. Ö. Polat, R. Korkusuz, and M. Berber, Hydroxychloroquine Use on Healthcare Workers Exposed to COVID-19 – A Pandemic Hospital Experience Sep 2020, Medical J. Bakirkoy, 280-6
208 patient HCQ prophylaxis study: 57% fewer cases (p=0.03).
Small prophylaxis study of 208 healthcare workers in Turkey, 138 with high-risk exposure received HCQ, while 70 with low and medium risk exposure did not. Covid-19 cases were lower in the treatment group, relative risk RR 0.43, p = 0.026. Since the control group had lower risk, the actual benefit may be larger. https://c19p.org/polat
65. L. Ayerbe, C. Risco-Risco, and S. Ayis, The association of treatment with hydroxychloroquine and hospital mortality in COVID-19 patients Sep 2020, Internal and Emergency Medicine, Volume 15, Issue 8, Page 1501-1506
LATE TREATMENT 2,075 patient HCQ late treatment study: 52% lower mortality (p=0.001).
2,075 hospital patients in Spain showing HCQ reduces mortality 52%, odds ratio OR 0.39, p<0.001, after adjustment for age, gender, temperature > 37 °C, and saturation of oxygen < 90% treatment with azithromycin, steroids, heparin, tocilizumab, a combination of lopinavir with ritonavir, and oseltamivir, and date of admission (See model 4). https://c19p.org/ayerbe
66. D. Pinato, A. Zambelli, J. Aguilar-Company, M. Bower, C. Sng, R. Salazar, A. Bertuzzi, J. Brunet, R. Mesia, E. Seguí, F. Biello, D. Generali, S. Grisanti, G. Rizzo, M. Libertini, A. Maconi, N. Harbeck, B. Vincenzi, R. Bertulli, D. Ottaviani, A. Carbó, R. Bruna, S. Benafif, A. Marrari, R. Wuerstlein, M. Carmona-Garcia, N. Chopra, C. Tondini, O. Mirallas, V. Tovazzi, M. Betti, S. Provenzano, V. Fotia, C. Cruz, A. Dalla Pria, F. D’Avanzo, J. Evans, N. Saoudi-Gonzalez, E. Felip, M. Galazi, I. Garcia-Fructuoso, A. Lee, T. Newsom-Davis, A. Patriarca, D. García-Illescas, R. Reyes, P. Dileo, R. Sharkey, Y. Wong, D. Ferrante et al., Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients Aug 2020, Cancer Discovery, Volume 10, Issue 10, Page 1465-1474
LATE TREATMENT 890 patient HCQ late treatment study: 59% lower mortality (p=0.0001).
Retrospective 890 cancer patients with Covid-19, adjusted mortality HR for HCQ/CQ 0.41, p<0.0001. Confirmed SARS-CoV-2 infection was required, which may help focus on more severe cases. Analysis with Cox proportional hazard model. Potential unmeasured confounders. https://c19p.org/pinato
67. B. Davido, G. Boussaid, I. Vaugier, T. Lansaman, F. Bouchand, C. Lawrence, J. Alvarez, P. Moine, V. Perronne, F. Barbot, A. Saleh-Mghir, C. Perronne, D. Annane, and P. De Truchis, Impact of medical care including anti-infective agents use on the prognosis of COVID-19 hospitalized patients over time Aug 2020, Int. J. Antimicrobial Agents, 2020, Volume 56, Issue 4, Page 106129
132 patient HCQ LATE TREATMENT study: 55% lower combined intubation/hospitalization (p=0.04).
Retrospective of 132 hospitalized patients. HCQ+AZ(52)/AZ(28) significantly reduced death/ICU, HR=0.45, p=0.04. Adjusted for Charlson Comorbidity Index (including age), obesity, O2, lymphocyte count, and treatments. Mean delay from admission to treatment 0.7 days. https://c19p.org/davido
68. S. Arshad, P. Kilgore, Z. Chaudhry, G. Jacobsen, D. Wang, K. Huitsing, I. Brar, G. Alangaden, M. Ramesh, J. McKinnon, W. O’Neill, M. Zervos, V. Nauriyal, A. Hamed, O. Nadeem, J. Swiderek, A. Godfrey, J. Jennings, J. Gardner-Gray, A. Ackerman, J. Lezotte, J. Ruhala, R. Fadel, A. Vahia, S. Gudipati, T. Parraga, A. Shallal, G. Maki, Z. Tariq, G. Suleyman, N. Yared, E. Herc, J. Williams, O. Lanfranco, P. Bhargava, and K. Reyes, Treatment with Hydroxychloroquine, Azithromycin, and Combination in Patients Hospitalized with COVID-19 Jun 2020, Int. J. Infect. Dis., July 1 2020, Volume 97, Page 396-403
LATE TREATMENT 2,541 patient HCQ late treatment study: 51% lower mortality (p=0.009).
HCQ decreases mortality from 26.4% to 13.5% (HCQ) or 20.1% (HCQ+AZ). Propensity matched HCQ HR 0.487, p=0.009. Michigan 2,541 patients retrospective. Before propensity matching the HCQ group average age is 5 years younger and the percentage of male patients is 4% higher which is likely to favor the treatment and the control respectively in the before-propensity matching results. Some reported limitations of this study are inaccurate. Corticosteroids were controlled for in the multivariate and propensity analyses as were age and comorbidities including cardiac disease and severity of illness. Age was an independent risk factor associated with mortality. HCQ was independently associated with decreased mortality, distinct from the steroid effect. 91% of all patients began treatment within two days of admission. HCQ was used throughout the study period, limiting time bias. Patients assigned to HCQ group had moderate and severe illness at presentation, which would favor worse outcome with HCQ. https://c19p.org/arshad
69. T. Mikami, H. Miyashita, T. Yamada, M. Harrington, D. Steinberg, A. Dunn, and E. Siau, Risk Factors for Mortality in Patients with COVID-19 in New York City Jun 2020, J. Gen. Intern. Med., Volume 36, Issue 1, Page 17-26
LATE TREATMENT 6,000 patient HCQ late treatment study: 47% lower mortality (p<0.0001).
HCQ decreases mortality, HR 0.53 (CI 0.41–0.67). IPTW adjustment does not significantly change HR 0.53 (0.41-0.68). Retrospective 6,000 patients in New York City. https://c19p.org/mikami
70. A. Ferreira, A. Oliveira‐e‐Silva, and P. Bettencourt, Chronic treatment with hydroxychloroquine and SARS-CoV-2 infection Jun 2020, J. Medical Virology, July 9, 2020, Volume 93, Issue 2, Page 755-759
26,815 patient HCQ prophylaxis study: 47% fewer cases (p<0.0001).
Chronic treatment with HCQ provides protection against Covid, odds ratio 0.51 (0.37-0.70). The actual benefit is likely to be larger because research shows that the risk of Covid-19 for systemic autoimmune disease patients is much higher overall. Ferri et al. show OR 4.42, p<0.001 which is the observed real-world risk, considering factors such as patients potentially being more careful to avoid exposure. https://c19p.org/ferreira
71. J. Lagier, M. Million, P. Gautret, P. Colson, S. Cortaredona, A. Giraud-Gatineau, S. Honoré, J. Gaubert, P. Fournier, H. Tissot-Dupont, E. Chabrière, A. Stein, J. Deharo, F. Fenollar, J. Rolain, Y. Obadia, A. Jacquier, B. La Scola, P. Brouqui, M. Drancourt, P. Parola, D. Raoult, S. Amrane, C. Aubry, M. Bardou, C. Berenger, L. Camoin-Jau, N. Cassir, C. Decoster, C. Dhiver, B. Doudier, S. Edouard, S. Gentile, K. Guillon-Lorvellec, M. Hocquart, A. Levasseur, M. Mailhe, I. Ravaux, M. Richez, Y. Roussel, P. Seng, C. Tomei, and C. Zandotti, Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis Jun 2020, Travel Med. Infect. Dis. 101791, Jun 25, 2020, Volume 36, Page 101791
LATE TREATMENT 3,737 patient HCQ late treatment study: 59% lower mortality (p=0.05).
Early treatment leads to significantly better clinical outcomes and faster viral load reduction. Matched sample mortality HR 0.41 p-value 0.048. Retrospective 3,737 patients. This study includes both outpatients and hospitalized patients. https://c19p.org/lagier
72. J. Sánchez-Álvarez, M. Fontán, C. Martín, M. Pelícano, C. Reina, Á. Prieto, E. Melilli, M. Barrios, M. Heras, and M. Pino, Status of SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN) Apr 2020, Nefrología, Volume 40, Issue 3, Page 272-278
LATE TREATMENT 375 patient HCQ late treatment study: 46% lower mortality (p=0.005).
Analysis of 868 patients on renal replacement therapy. Statistically significant reduction in mortality with HCQ for patients on dialysis (OR 0.47, p=0.005). No statistically significant change was found for transplant patients (the result is not given but likely the sample size is too small – the number of transplant patients was half the number of dialysis patients). https://c19p.org/sanchezalvarez
73. R. Esper, R. Souza da Silva, F. Teiichi, C. Oikawa, M. Castro, A. Razuk-Filho, P. Batista, S. Lotze, C. Nunes da Rocha, R. Filho, S. Barbosa de Oliveira, P. Ribeiro, V. Martins, F. Bueno, P. Esper, E. Parrillo, Empirical treatment with hydroxychloroquine and azithromycin for suspected cases of COVID-19 followed-up by telemedicine Apr 2020, Prevent Senior Institute, São Paulo, Brazil
636 patient HCQ early treatment study: 64% lower hospitalization (p=0.02).
636 patients. HCQ+AZ reduced hospitalization 79% when used within 7 days (65% overall). Non-randomized. https://c19p.org/esper
74. A. Agusti, E. Guillen, A. Ayora, A. Anton, C. Aguilera, X. Vidal, C. Andres, M. Alonso, M. Espuga, J. Esperalba, M. Gorgas, B. Almirante, and E. Ribera, Efficacy and safety of hydroxychloroquine in healthcare professionals with mild SARS-CoV-2 infection: prospective, non-randomized trial Dec 2020, Enfermedades Infecciosas y Microbiología Clínica, Volume 40, Issue 6, Page 289-295
142 patient HCQ early treatment study: 68% lower progression (p=0.21) and 32% faster viral clearance.
Small trial of low dose HCQ for healthcare workers with mild SARS-CoV-2 showing 68% lower progression to pneumonia, p = 0.21, and faster, but not statistically significant viral clearance. There were no ICU admissions or deaths. Prospective non-randomized study. https://c19p.org/agusti
75. A. Heberto, P. Carlos, C. Antonio, P. Patricia, T. Enrique, M. Danira, G. Benito, and M. Alfredo, Implications of myocardial injury in Mexican hospitalized patients with coronavirus disease 2019 (COVID-19) Sep 2020, IJC Heart & Vasculature, Volume 30, Page 100638
LATE TREATMENT 254 patient HCQ late treatment study: 54% lower mortality (p=0.04) and 65% lower ventilation (p=0.008).
Observational prospective 254 hospitalized patients, HCQ+AZ mortality odds ratio OR 0.36, p = 0.04. Ventilation OR 0.20, p = 0.008. https://c19p.org/heberto
76. P. Gautret, J. Lagier, P. Parola, V. Hoang, L. Meddeb, M. Mailhe, B. Doudier, J. Courjon, V. Giordanengo, V. Vieira, H. Tissot Dupont, S. Honoré, P. Colson, E. Chabrière, B. La Scola, J. Rolain, P. Brouqui, and D. Raoult, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial Mar 2020, Int. J. of Antimicrobial Agents, Volume 56, Issue 1, Page 105949
EARLY TREATMENT 36 patient HCQ early treatment study: 66% improved viral clearance (p=0.001).
HCQ was significantly associated with reduction/elimination of viral load, which was enhanced with AZ. Analysis of this paper have raised methodological issues. This study should be viewed in the context of the broader positive outcomes in a multitude of other studies. An update to this paper, including originally excluded patients, confirms the effectiveness of HCQ+AZ on viral clearance and early discharge. Authors responsive to outside methodology and data queries, unlike other HCQ authors. https://c19p.org/gautretjaa
77. A. Ouédraogo, G. Bougma, A. Baguiya, A. Sawadogo, P. Kaboré, C. Minougou, A. Diendéré, S. Maiga, C. Agbaholou, A. Hema, A. Sondo, G. Ouédraogo, A. Sanou, and M. Ouedraogo, Factors associated with the occurrence of acute respiratory distress and death in patients with COVID-19 in Burkina Faso Feb 2021, Revue des Maladies Respiratoires, Volume 38, Issue 3, Page 240-248
LATE TREATMENT 456 patient HCQ late treatment study: 33% lower mortality (p=0.38) and 68% lower severe cases (p=0.001).
Retrospective 456 patients in Burkina Faso showing lower risk of acute respiratory distress syndrome (p=0.001) and mortality (p=0.38) with HCQ. https://c19p.org/ouedraogo
78. D. Dhibar, N. Arora, A. Kakkar, N. Singla, R. Mohindra, V. Suri, A. Bhalla, N. Sharma, M. Singh, A. Prakash, L. PVM, and B. Medhi, Post Exposure Prophylaxis with Hydroxychloroquine (HCQ) for the Prevention of COVID-19, a Myth or a Reality? The PEP-CQ Study Nov 2020, Int. J. Antimicrobial Agents, Volume 56, Issue 6, Page 106224
317 patient HCQ prophylaxis study: 44% fewer symptomatic cases (p=0.21) and 50% fewer cases (p=0.04).
Low-dose prospective PEP study with 132 HCQ patients and 185 control patients, showing significantly lower Covid-19 cases with treatment. There were no serious adverse events. HCQ 800mg on day one followed by 400mg once weekly for 3 weeks. https://c19p.org/dhibar
79. K. Atipornwanich, S. Kongsaengdao, P. Harnsomburana, R. Nanna, C. Chtuparisute, P. Saengsayan, K. Bangpattanasiri, W. Manosuthi, N. Sawanpanyalert, A. Srisubat, S. Thanasithichai, B. Maneeton, N. Maneeton, C. Suthisisang, J. Pratuangdejkul, and S. Akksilp, Various Combinations of Favipiravir, Lopinavir-Ritonavir, Darunavir-Ritonavir, High-Dose Oseltamivir, and Hydroxychloroquine for the Treatment of COVID-19: A Randomized Controlled Trial (FIGHT-COVID-19 Study) Oct 2021, SSRN Electronic J.
LATE TREATMENT 200 patient HCQ late treatment RCT: 56% lower mortality (p=0.07), 54% lower progression (p=0.02), and 7% faster viral clearance (p=0.51).
RCT 320 patients in Thailand, showing significantly lower progression with HCQ for moderate/severe patients, and faster viral clearance with mild patients (statistically significant for 800mg). There are two sets of results – for moderate/severe patients, and for mild patients. There was no mortality for mild patients. https://c19p.org/atipornwanich
80. M. Goenka, S. Afzalpurkar, U. Goenka, S. Das, M. Mukherjee, S. Jajodia, B. Shah, V. Patil, G. Rodge, U. Khan, and S. Bandyopadhyay, Seroprevalence of COVID-19 Amongst Health Care Workers in a Tertiary Care Hospital of a Metropolitan City from India Oct 2020, SSRN
962 patient HCQ prophylaxis study: 87% lower IgG positivity (p=0.03).
Study of SARS-CoV-2-IgG antibodies in 1122 health care workers in India finding 87% lower positives for adequate HCQ prophylaxis, 1.3% HCQ versus 12.3% for no HCQ prophylaxis. Adequate prophylaxis is defined as 400mg 1/wk for >6 weeks. https://c19p.org/goenka
81. M. Lyngbakken, J. Berdal, A. Eskesen, D. Kvale, I. Olsen, C. Rueegg, A. Rangberg, C. Jonassen, T. Omland, H. Røsjø, and O. Dalgard, A pragmatic randomized controlled trial reports lack of efficacy of hydroxychloroquine on coronavirus disease 2019 viral kinetics Jul 2020, Nature Communications, Volume 11, Issue 1
LATE TREATMENT 53 patient HCQ late treatment RCT: 4% lower mortality (p=1) and 71% improved viral reduction rate (p=0.51).
Small RCT of nasopharyngeal viral load not showing significant differences. The rate of reduction for HCQ was 0.24 [0.03-0.46] RNA copies/mL/24h, and 0.14 [-0.10-0.37] for the control group (71% faster with HCQ but not statistically significant with the small sample size of 27 HCQ and 26 control patients). Analysis only over 96 hours. https://c19p.org/lyngbakken
82. R. Bhattacharya, S. Chowdhury, R. Mukherjee, M. Kulshrestha, R. Ghosh, S. Saha, and A. Nandi, Pre exposure Hydroxychloroquine use is associated with reduced COVID19 risk in healthcare workers Jun 2020, medRxix
106 patient HCQ prophylaxis study: 81% fewer cases (p=0.001).
HCQ reduced cases from 38% to 7%. 106 people. No serious adverse effects. https://c19p.org/bhattacharya
83. Zhong Nanshan (钟南山) et al., Efficacy and safety of chloroquine for treatment of COVID-19. An open-label, multi-center, non-randomized trial Mar 2020, Zhong Nanshan
LATE TREATMENT 197 patient HCQ late treatment study: 80% improved viral clearance (p=0.0001).
197 patients. CQ effective. Day 10 viral RNA negative 91.4% HCQ versus 57.4% control. Median time to negative test 3 days versus 9 days for control. https://c19p.org/zhong2
84. C. Isnardi, K. Roberts, V. Saurit, I. Petkovic, R. Báez, R. Quintana, Y. Tissera, S. Ornella, M. D.Angelo Exeni, C. Pisoni, V. Castro Coello, G. Berbotto, M. Haye Salinas, E. Velozo, Á. Reyes Torres, R. Tanten, M. Zelaya, C. Gobbi, C. Alonso, M. De los Ángeles Severina, F. Vivero, A. Paula, A. Cogo, G. Alle, M. Pera, R. Nieto, M. Cosatti, C. Asnal, D. Pereira, J. Albiero, V. Savio, F. Maldonado, M. Gamba, N. Germán, A. Baños, J. Gallino Yanzi, M. Gálvez Elkin, J. Morbiducci, M. Martire, H. Maldonado Ficco, M. Schmid, J. Villafañe Torres, M. De los Ángeles Correa, M. Medina, M. Cusa, J. Scafati, S. Agüero, N. Lloves Schenone, E. Soriano, C. Graf et al., Sociodemographic and clinical factors associated with poor COVID-19 outcomes in patients with rheumatic diseases: data from the SAR-COVID Registry Oct 2022, Clinical Rheumatology
2,066 patient HCQ prophylaxis study: 34% lower mortality (p=0.23), 48% lower severe cases (p=0.02), and 17% lower hospitalization (p=0.09).
Retrospective 1,915 rheumatic disease patients with Covid-19 in Argentina, showing lower mortality, severe oxygen requirement, and hospitalization with CQ/HCQ (antimalarial) use in unadjusted results, statistically significant only for severe oxygen requirement. https://c19p.org/isnardi
85. E. Sobngwi, S. Zemsi, M. Guewo, J. Katte, C. Kouanfack, L. Mfeukeu, A. Zemsi, Y. Wasnyo, A. Ntsama Assiga, A. Ndi Manga, J. Sobngwi-Tambekou, W. Ngatchou, C. Moussi Omgba, J. Mbanya, P. Ongolo Zogo, and P. Fouda, Doxycycline vs Hydroxychloroquine + Azithromycin in the Management of COVID-19 Patients: An Open-Label Randomized Clinical Trial in Sub-Saharan Africa (DOXYCOV) Jul 2021, Cureus
EARLY TREATMENT 187 patient HCQ early treatment RCT: 52% improved recovery (p=0.44) and 3% improved viral clearance (p=0.88).
RCT 194 mild/asymptomatic low-risk patients in Cameroon, 97 treated with HCQ+AZ and 97 treated with doxycycline, showing 2.1% symptomatic patients at day 10 with HCQ+AZ, versus 4.3% with doxycycline, without statistical significance. There were only 6 patients with symptoms at day 10. There was no mortality or hospitalization, and no major adverse events. https://c19p.org/sobngwi
86. P. Sivapalan, C. Ulrik, T. Lapperre, R. Bojesen, J. Eklöf, A. Browatzki, J. Wilcke, V. Gottlieb, K. Håkansson, C. Tidemandsen, O. Tupper, H. Meteran, C. Bergsøe, E. Brøndum, U. Bødtger, D. Bech Rasmussen, S. Graff Jensen, L. Pedersen, A. Jordan, H. Priemé, C. Søborg, I. Steffensen, D. Høgsberg, T. Klausen, M. Frydland, P. Lange, A. Sverrild, M. Ghanizada, F. Knop, T. Biering-Sørensen, J. Lundgren, and J. Jensen, Azithromycin and hydroxychloroquine in hospitalised patients with confirmed COVID-19–a randomised double-blinded placebo-controlled trial Jun 2021, European Respiratory J., Volume 59, Issue 1, Page 2100752
LATE TREATMENT 117 patient HCQ late treatment RCT: 92% lower mortality (p=0.32), 22% higher ICU admission (p=1), and 8% lower hospital discharge (p=0.36).
Early terminated late stage (8 days from onset, 59% on oxygen) RCT not showing statistically significant differences. https://c19p.org/sivapalan
87. A. Omrani, S. Pathan, S. Thomas, T. Harris, P. Coyle, C. Thomas, I. Qureshi, Z. Bhutta, N. Mawlawi, R. Kahlout, A. Elmalik, A. Azad, J. Daghfal, M. Mustafa, A. Jeremijenko, H. Soub, M. Khattab, M. Maslamani, and S. Thomas, Randomized double-blinded placebo-controlled trial of hydroxychloroquine with or without azithromycin for virologic cure of non-severe Covid-19 Nov 2020, EClinicalMedicine, Volume 29-30, Page 100645
EARLY TREATMENT 456 patient HCQ early treatment RCT: 12% lower hospitalization (p=1), 26% improved recovery (p=0.58), and 10% worse viral clearance (p=0.13).
Low-risk patient RCT for HCQ+AZ and HCQ vs. control, not showing any significant differences. Authors note that the results are not applicable to higher risk patients, that positive PCR may simply reflect detection of inactive (non-infectious) viral remnants, that an alternative dosage regimen may be more effective, and that medication adherence was unknown. HCQ dosing was 600mg/day for 1 week, therapeutic levels may not be reached for several days. There were no deaths or serious adverse events. Viral load was already very high at baseline. https://c19p.org/omrani
88. T. Korkmaz, A. Şener, V. Gerdan, and İ. Kızıloglu, The effect of Hydroxychloroquine use due to rheumatic disease on the risk of Covid-19 infection and its course May 2021, Authorea
694 patient HCQ prophylaxis study: 82% lower mortality (p=0.19) and 94% fewer cases (p<0.0001).
Retrospective 683 patients in a rheumatology department, 384 chronic HCQ users and 299 control patients, showing no mortality for HCQ users vs. 2 deaths in the control group, and significantly fewer cases for HCQ users. https://c19p.org/korkmaz
89. J. Finkelstein and X. Huo, The Efficacy of Long-Term Hydroxychloroquine Use in the Prevention of COVID-19: A Retrospective Cohort Study Jun 2023, Studies in Health Technology and Informatics
110,038 patient HCQ prophylaxis PSM study: 21% fewer cases (p=0.0007).
PSM retrospective SLE/RA patients in the USA, showing lower Covid-19 cases with HCQ prophylaxis. https://c19p.org/finkelstein
90. N. AlQadheeb, H. AlMubayedh, S. AlBadrani, A. Salam, M. AlOmar, A. AlAswad, M. AlMualim, Z. AlQamariat, and R. AlHubail, Impact of common comorbidities on antimicrobial consumption and mortality amongst critically ill COVID-19 patients: A retrospective two center study in Saudi Arabia May 2023, Clinical Infection in Practice, Volume 19, Page 100229
LATE TREATMENT 848 patient HCQ ICU study: 35% lower mortality (p=0.0001).
Retrospective 848 ICU patients in Saudi Arabia, showing lower mortality with HCQ in unadjusted results. https://c19p.org/alqadheeb
91. Ş. Bubenek-Turconi, S. Andrei, L. Văleanu, M. Ştefan, I. Grigoraş, S. Copotoiu, C. Bodolea, D. Tomescu, M. Popescu, D. Filipescu, H. Moldovan, A. Rogobete, C. Bălan, B. Moroşanu, D. Săndesc, and R. Arafat, Clinical characteristics and factors associated with ICU mortality during the first year of the SARS-Cov-2 pandemic in Romania Nov 2022, European J. Anaesthesiology, Volume Publish Ahead of Print
LATE TREATMENT HCQ ICU study: 22% lower mortality (p=0.01).
Prospective study of 9,058 Covid-19 ICU patients in Romania, showing lower mortality with HCQ treatment. https://c19p.org/bubenekturconi
92. R. Go and T. Nyirenda, Hydroxychloroquine, azithromycin and methylprednisolone and in hospital survival in severe COVID-19 pneumonia Sep 2022, Frontiers in Pharmacology, Volume 13
LATE TREATMENT HCQ late treatment study: 55% lower mortality (p=0.03).
Retrospective 759 hospitalized patients in the USA, showing lower mortality with combined HCQ+AZ+methylprednisolone treatment compared to methylprednisolone monotherapy. https://c19p.org/go2
93. A. Bowen, J. Zucker, Y. Shen, S. Huang, Q. Yan, M. Annavajhala, A. Uhlemann, L. Kuhn, M. Sobieszczyk, and D. Castor, Reduction in risk of death among patients admitted with COVID-19 between first and second epidemic waves in New York City Aug 2022, Open Forum Infectious Diseases
LATE TREATMENT 4,631 patient HCQ late treatment study: 20% lower mortality (p=0.007).
Retrospective 4,631 hospitalized patients in New York, showing higher mortality with remdesivir, and lower mortality with HCQ. Authors suggest that increased mortality during the first epidemic wave was partly due to strain on hospital resources, which might have been avoided with Trump’s proposal for HCQ in eligible populations. https://c19p.org/bowen
94. A. Yadav, A. Kotwal, and S. Ghosh, Hydroxychloroquine/chloroquine prophylaxis among health-care workers: Was it really preventive? – Evidence from a multicentric cross-sectional study Jul 2022, Indian J. Community Medicine, Volume 47, Issue 2, Page 202
2,224 patient HCQ prophylaxis study: 20% lower seropositivity (p=0.1).
Retrospective 2,224 healthcare workers in India, showing lower risk of seropositivity with HCQ prophylaxis, without statistical significance. https://c19p.org/yadav4
95. M. Ebongue, D. Lemogoum, L. Endale-Mangamba, B. Barche, C. Eyoum, S. Simo Yomi, D. Mekolo, V. Ngambi, J. Doumbe, C. Sike, J. Boombhi, G. Ngondi, C. Biholong, J. Kamdem, L. Mbenoun, C. Tegeu, A. Djomou, A. Dzudie, F. Kamdem, F. Ntock, L. Mfeukeu, E. Sobngwi, I. Penda, R. Njock, N. Essomba, J. Yombi, and W. Ngatchou, Factors predicting in-hospital all-cause mortality in COVID 19 patients at the Laquintinie Hospital Douala, Cameroon Mar 2022, Travel Medicine and Infectious Disease, Page 102292
LATE TREATMENT 580 patient HCQ late treatment study: 43% lower mortality (p=0.04).
Retrospective 580 hospitalized Covid+ patients in Cameroon, showing lower mortality with HCQ+AZ treatment. https://c19p.org/ebongue
96. C. Lavilla Olleros, C. Ausín García, A. Bendala Estrada, A. Muñoz, P. Wikman Jogersen, A. Fernández Cruz, V. Giner Galvañ, J. Vargas, J. Seguí Ripoll, M. Rubio-Rivas, R. Miranda Godoy, L. Mérida Rodrigo, E. Fonseca Aizpuru, F. Arnalich Fernández, A. Artero, J. Loureiro Amigo, G. García García, L. Corral Gudino, J. Jiménez Torres, J. Casas-Rojo, and J. Millán Núñez-Cortés, Use of glucocorticoids megadoses in SARS-CoV-2 infection in a spanish registry: SEMI-COVID-19 Jan 2022, PLOS ONE, Volume 17, Issue 1, Page e0261711
LATE TREATMENT 14,921 patient HCQ late treatment study: 36% lower mortality (p<0.0001).
Retrospective 14,921 hospitalized patients in Spain, showing lower mortality with HCQ treatment. https://c19p.org/lavillaolleros
97. J. McKinnon, D. Wang, M. Zervos, M. Saval, L. Marshall-Nightengale, P. Kilgore, P. Pabla, E. Szandzik, K. Maksimowicz-McKinnon, and W. O’Neill, Safety and Tolerability of Hydroxychloroquine in healthcare workers and first responders for the prevention of COVID-19: WHIP COVID-19 Study Dec 2021, Int. J. Infectious Diseases
543 patient HCQ prophylaxis RCT: 2% fewer symptomatic cases (p=1) and 51% fewer cases (p=0.6).
HCQ prophylaxis RCT with 201 weekly HCQ patients, 197 daily HCQ patients, and 200 control patients, concluding the prophylaxis is safe. There were no grade 3 or 4 AEs, SAEs, ER visits, or hospitalizations. There were only 4 confirmed cases, 2 in the placebo arm and one in each HCQ arm. 60% of patients had exposure at baseline. HCQ 400mg weekly or HCQ 200mg daily after a loading dose of 400mg on day 1. https://c19p.org/mckinnon
98. P. Panda, B. Singh, B. Moirangthem, Y. Bahurupi, S. Saha, G. Saini, M. Dhar, M. Bairwa, V. Pai, A. Agarwal, G. Sindhwani, S. Handu, and R. Kant, Antiviral Combination Clinically Better Than Standard Therapy in Severe but Not in Non-Severe COVID-19 Sep 2021, Clinical Pharmacology: Advances and Applications, Volume 13, Page 185-195
LATE TREATMENT 41 patient HCQ late treatment RCT: 48% lower mortality (p=0.45).
RCT 111 patients in India in 5 groups: severe patients: a) standard treatment, b) hydroxychloroquine + ribavirin + standard treatment, or c) lopinavir + ritonavir + ribavirin + standard treatment, and non-severe: a) standard treatment or b) hydroxychloroquine + ribavirin. Non-severe patients were transferred to the severe group on progression. https://c19p.org/panda2
99. S. Naggie, A. Milstone, M. Castro, S. Collins, S. Lakshmi, D. Anderson, L. Cahuayme-Zuniga, K. Turner, L. Cohen, J. Currier, E. Fraulo, A. Friedland, J. Garg, A. George, H. Mulder, R. Olson, E. O’Brien, R. Rothman, E. Shenkman, J. Shostak, C. Woods, K. Anstrom, and A. Hernandez, Hydroxychloroquine for pre-exposure prophylaxis of COVID-19 in health care workers: A randomized, multicenter, placebo-controlled trial (HERO-HCQ) Aug 2021, Int. J. Infectious Diseases
1,359 patient HCQ prophylaxis RCT: 24% fewer symptomatic cases (p=0.18).
HCQ prophylaxis RCT reporting statistically significant lower cases when pooling results with the Covid PREP RCT, OR 0.74 [0.55-1.0] p = 0.046. There were no significant safety issues. The trials were both terminated early resulting in a loss of power; however the combination shows statistically significant efficacy of HCQ. Note that this result has been censored in the journal version, see original in medrxiv.org HERE. The journal paper still shows the Covid PREP paper in the reference list, but the analysis and discussion has been deleted. The journal version falsely states: “The prophylactic use of HCQ by HCW was safe but not effective” whereas the paper actually estimates OR 0.75, which becomes statistically significant OR 0.74 when pooled with Covid PREP. The preprint contains a different version: “…but did not produce a clinically useful treatment.” It’s unclear why ~25% fewer cases would not be useful. They also state “This is one of several negative studies” however the result is positive, just not reaching statistical significance before pooling with Covid PREP. This same author (Susanna Naggie, MD) published a highly questionable, poorly conducted study on ivermectin. More issues with ivermectin discussed here and here: https://c19p.org/naggie
100. F. Taieb, K. Mbaye, B. Tall, N. Lakhe, C. Talla, D. Thioub, A. Ndoye, D. Ka, A. Gaye, V. Cissé Diallo, N. Dia, P. Ba, M. Cissé, M. Diop, C. Diagne, L. Fortes, M. Diop, N. Fall, F. Sarr, M. Diatta, M. Barry, A. Badiane, A. Seck, P. Dubrous, O. Faye, I. Vigan-Womas, C. Loucoubar, A. Sall, and M. Seydi, Hydroxychloroquine and Azithromycin Treatment of Hospitalized Patients Infected with SARS-CoV-2 in Senegal from March to October 2020 Jun 2021, J. Clin. Med. 2021, Volume 10, Issue 13, Page 2954
LATE TREATMENT 926 patient HCQ late treatment study: 39% higher hospital discharge (p=0.02).
Retrospective 926 patients in Senegal, 674 treated with HCQ+AZ, showing significantly higher hospital discharge at day 15 with treatment. https://c19p.org/taieb
101. J. Lagier, M. Million, S. Cortaredona, L. Delorme, P. Colson, P. Fournier, P. Brouqui, D. Raoult, and P. Parola, Outcomes of 2,111 COVID-19 hospitalised patients treated with 2 hydroxychloroquine/azithromycin and other regimens in Marseille, France: a 3 monocentric retrospective analysis Jun 2021, Therapeutics and Clinical Risk Management, Volume 18, Page 603-617
LATE TREATMENT 2,111 patient HCQ late treatment study: 32% lower mortality (p=0.004).
Retrospective 2,011 hospitalized patients in France, median age 67, showing lower mortality with HCQ+AZ, and further benefit with the addition of zinc. https://c19p.org/lagier2
102. F. De Rosa, A. Palazzo, T. Rosso, N. Shbaklo, M. Mussa, L. Boglione, E. Borgogno, A. Rossati, S. Mornese Pinna, S. Scabini, G. Chichino, S. Borrè, V. Del Bono, P. Garavelli, D. Barillà, F. Cattel, G. Di Perri, G. Ciccone, T. Lupia, and S. Corcione, Risk Factors for Mortality in COVID-19 Hospitalized Patients in Piedmont, Italy: Results from the Multicenter, Regional, CORACLE Registry Apr 2021, J. Clin. Med., Volume 10, Issue 9, Page 1951
LATE TREATMENT 1,538 patient HCQ late treatment study: 35% lower mortality (p=0.02).
Retrospective 1,538 hospitalized patients in Italy, showing only HCQ associated with reduced mortality. Authors analyze mortality amongst those that were alive at day 7 to avoid survival time bias due to drug recording requiring a minimum of 5 days treatment. https://c19p.org/derosa
103. Z. Alzahrani, K. Alghamdi, and A. Almaqati, Clinical characteristics and outcome of COVID-19 in patients with rheumatic diseases Apr 2021, Rheumatology Int. , Volume 41, Issue 6, Page 1097-1103
47 patient HCQ prophylaxis study: 59% lower mortality (p=1), 81% lower ventilation (p=0.54), and 33% lower severe cases (p=0.7).
Retrospective 47 rheumatic disease patients not finding significant differences with HCQ. https://c19p.org/alzahrani
104. N. Dev, R. Meena, D. Gupta, N. Gupta, and J. Sankar, Risk factors and frequency of COVID-19 among healthcare workers at a tertiary care centre in India: a case–control study Mar 2021, Transactions of The Royal Society of Tropical Medicine and Hygiene, Volume 115, Issue 5, Page 551-556
759 patient HCQ prophylaxis study: 26% fewer cases (p=0.003).
Retrospective case control study of 3,100 healthcare workers in India showing lower cases with HCQ prophylaxis, and an inverse association between the number of HCQ doses taken and the risk of Covid-19 cases. Low risk population with no mortality and no severe cases. https://c19p.org/dev
105. F. Taccone, N. Van Goethem, R. De Pauw, X. Wittebole, K. Blot, H. Van Oyen, T. Lernout, M. Montourcy, G. Meyfroidt, and D. Van Beckhoven, The role of organizational characteristics on the outcome of COVID-19 patients admitted to the ICU in Belgium Dec 2020, The Lancet Regional Health – Europe, Volume 2, Page 100019
LATE TREATMENT 1,747 patient HCQ ICU study: 25% lower mortality (p=0.02).
Retrospective 1,747 ICU patients in Belgium showing lower mortality with HCQ, multivariate mixed effects analysis HCQ adjusted odds ratio 0.64 [0.45-0.92]. https://c19p.org/taccone
106. J. Tan, Y. Yuan, C. Xu, C. Song, D. Liu, D. Ma, and Q. Gao, A retrospective comparison of drugs against COVID-19 Dec 2020, Virus Research, Volume 294, Page 198262
LATE TREATMENT 285 patient HCQ late treatment study: 35% shorter hospitalization (p=0.04).
Retrospective 333 patients in China, with only 8 HCQ patients, showing shorter duration of hospitalization with HCQ. https://c19p.org/tan2
107. S. Szente Fonseca, A. De Queiroz Sousa, A. Wolkoff, M. Moreira, B. Pinto, C. Valente Takeda, E. Rebouças, A. Vasconcellos Abdon, A. Nascimento, and H. Risch, Risk of Hospitalization for Covid-19 Outpatients Treated with Various Drug Regimens in Brazil: Comparative Analysis Oct 2020, Travel Medicine and Infectious Disease, Volume 38, Page 101906
EARLY TREATMENT 717 patient HCQ early treatment study: 64% lower hospitalization (p=0.0008).
64% lower hospitalization with HCQ. Retrospective 717 patients in Brazil with early treatment, adjusted OR 0.32, p=0.00081, for HCQ versus no medication, and OR 0.45, p=0.0065, for HCQ vs. various other treatments. https://c19p.org/fonseca
108. A. Lammers, R. Brohet, R. Theunissen, C. Koster, R. Rood, D. Verhagen, K. Brinkman, R. Hassing, A. Dofferhoff, R. El Moussaoui, G. Hermanides, J. Ellerbroek, N. Bokhizzou, H. Visser, M. Van den Berge, H. Bax, D. Postma, and P. Groeneveld, Early hydroxychloroquine but not chloroquine use reduces ICU admission in COVID-19 patients Sep 2020, Int. J. Infectious Diseases, Volume 101, Page 283-289
LATE TREATMENT 1,064 patient HCQ late treatment study: 32% lower combined mortality/ICU admission (p=0.02).
Observational study 1,064 hospitalized patients in the Netherlands, 53% reduced risk of transfer to the ICU for mechanical ventilation with HCQ treatment starting on the first day of admission. Weighted propensity score adjusted hazard ratio for transfer to the ICU with HCQ treatment, HR = 0.47, p = 0.008. For CQ, HR = 0.8, p = 0.207. Mortality results in this study are only for mortality before transfer to the ICU. The combined ICU/death HR was 0.68, p = 0.024 for HCQ, and 0.85, p = 0.224 for CQ. Observational, multicenter, cohort study of hospitalized COVID-19 patients. 189 HCQ patients, 377 CQ, 498 control. https://c19p.org/lammers
109. M. Ashinyo, V. Duti, S. Dubik, K. Amegah, S. Kutsoati, E. Oduro-Mensah, P. Puplampu, M. Gyansa-Lutterodt, D. Darko, K. Buabeng, A. Ashinyo, A. Ofosu, N. Baddoo, S. Akoriyea, F. Ofei, and P. Kuma-Aboagye, Clinical characteristics, treatment regimen and duration of hospitalization among COVID-19 patients in Ghana: a retrospective cohort study Sep 2020, Pan African Medical J., Volume 37
LATE TREATMENT 307 patient HCQ late treatment study: 33% shorter hospitalization (p=0.03).
Retrospective 307 hospital patients in Ghana showing 33% reduction in hospitalization time with HCQ, 29% reduction with HCQ+AZ, and 37% reduction with CQ+AZ. https://c19p.org/ashinyo
110. A. Castelnuovo, S. Costanzo, A. Antinori, N. Berselli, L. Blandi, R. Bruno, R. Cauda, G. Guaraldi, L. Menicanti, I. My, G. Parruti, G. Patti, S. Perlini, F. Santilli, C. Signorelli, E. Spinoni, G. Stefanini, A. Vergori, W. Ageno, A. Agodi, L. Aiello, P. Agostoni, S. Moghazi, M. Astuto, F. Aucella, G. Barbieri, A. Bartoloni, M. Bonaccio, P. Bonfanti, F. Cacciatore, L. Caiano, F. Cannata, L. Carrozzi, A. Cascio, A. Ciccullo, A. Cingolani, F. Cipollone, C. Colomba, F. Crosta, C. Pra, G. Danzi, D. D’Ardes, K. Donati, P. Giacomo, F. Gennaro, G. Di Tano, G. D’Offizi, T. Filippini, F. Fusco, I. Gentile et al., Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study Aug 2020, European J. Internal Medicine, Volume 82, Page 38-47
LATE TREATMENT 3,451 patient HCQ late treatment study: 30% lower mortality (p<0.0001).
Retrospective 3,451 hospitalized patients, 30% reduction in mortality with HCQ after propensity adjustment, HR 0.70 [0.59 – 0.84]. https://c19p.org/dicastelnuovo
111. L. Catteau, N. Dauby, M. Montourcy, E. Bottieau, J. Hautekiet, E. Goetghebeur, S. Van Ierssel, E. Duysburgh, H. Van Oyen, C. Wyndham-Thomas, D. Van Beckhoven, K. Bafort, L. Belkhir, N. Bossuyt, P. Caprasse, V. Colombie, P. De Munter, J. Deblonde, D. Delmarcelle, M. Delvallee, R. Demeester, T. Dugernier, X. Holemans, B. Kerzmann, P. Yves Machurot, P. Minette, J. Minon, S. Mokrane, C. Nachtergal, S. Noirhomme, D. Piérard, C. Rossi, C. Schirvel, E. Sermijn, F. Staelens, F. Triest, N. Goethem, J. Praet, A. Vanhoenacker, R. Verstraete, and E. Willems, Low-dose Hydroxychloroquine Therapy and Mortality in Hospitalized Patients with COVID-19: A Nationwide Observational Study of 8075 Participants Aug 2020, Int. J. Antimicrobial Agents, Volume 56, Issue 4, Page 106144
LATE TREATMENT 8,075 patient HCQ late treatment study: 32% lower mortality (p<0.0001).
Retrospective 8,075 hospitalized patients, 4,542 low-dose HCQ, 3,533 control. 35% lower mortality for HCQ (17.7% vs. 27.1%), adjusted HR 0.68 [0.62–0.76]. Low-dose HCQ monotherapy was independently associated with lower mortality in hospitalized patients. Patients exposed to others therapies (TCZ, AZ, LPV/RTV) were excluded. Statistical analysis was performed by an independent group. Calendar time of prescription and immortal time bias was taken into account. Corticosteroids prescriptions was low in both groups. https://c19p.org/catteau
112. C. Chen, Y. Lin, T. Chen, T. Tseng, H. Wong, C. Kuo, W. Lin, S. Huang, W. Wang, J. Liao, C. Liao, Y. Hung, T. Lin, T. Chang, C. Hsiao, Y. Huang, W. Chung, C. Cheng, and S. Cheng, A Multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate Coronavirus disease 2019 (COVID-19) Jul 2020, PLoS ONE, Volume 15, Issue 12, Page e0242763
LATE TREATMENT 33 patient HCQ late treatment RCT: 24% improved viral clearance (p=0.71).
2 very small studies with hospitalized patients in Taiwan. RCT with 21 treatment and 12 standard-of-care patients. No mortality, or serious adverse effects. Median time to negative RNA 5 days versus 10 days standard-of-care, p=0.4. Risk of PCR+ at day 14, RR 0.76, p = 0.71. Small retrospective study with 12 of 28 HCQ patients and 5 of 9 in the control group being PCR- at day 14, RR 1.29, p = 0.7. The RCT and retrospective studies are listed separately. https://c19p.org/chen25
113. W. Tang, Z. Cao, M. Han, Z. Wang, J. Chen, W. Sun, Y. Wu, W. Xiao, S. Liu, E. Chen, W. Chen, X. Wang, J. Yang, J. Lin, Q. Zhao, Y. Yan, Z. Xie, D. Li, Y. Yang, L. Liu, J. Qu, G. Ning, G. Shi, and Q. Xie, Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial Apr 2020, BMJ 2020, 369, Page m1849
LATE TREATMENT 150 patient HCQ late treatment RCT: 21% improved viral clearance (p=0.51).
150 patient very late stage RCT showing no significant difference. Treatment was very late, an average of 16.6 days after symptom onset. Data favorable to HCQ was deleted in the second version, see analysis here. “[HCQ] accelerate[s] the alleviation of clinical symptoms”; “More rapid alleviation of clinical symptoms with standard-of-care plus HCQ than with standard-of-care alone was observed during the second week since randomization”; “The efficacy of HCQ on the alleviation of symptoms, HR 8.83 [1.09-71.3], was more evident when the confounding effects of other anti-viral agents were removed.” https://c19p.org/tang
114. O. Mitjà, M. Corbacho-Monné, M. Ubals, C. Tebé, J. Peñafiel, A. Tobias, E. Ballana, A. Alemany, N. Riera-Martí, C. Pérez, C. Suñer, P. Laporte, P. Admella, J. Mitjà, M. Clua, L. Bertran, M. Sarquella, S. Gavilán, J. Ara, J. Argimon, J. Casabona, G. Cuatrecasas, P. Cañadas, A. Elizalde-Torrent, R. Fabregat, M. Farré, A. Forcada, G. Flores-Mateo, E. Muntada, N. Nadal, S. Narejos, A. Nieto, N. Prat, J. Puig, C. Quiñones, J. Reyes-Ureña, F. Ramírez-Viaplana, L. Ruiz, E. Riveira-Muñoz, A. Sierra, C. Velasco, R. Vivanco-Hidalgo, A. Sentís, C. G-Beiras, B. Clotet, and M. Vall-Mayans, Hydroxychloroquine for Early Treatment of Adults with Mild Covid-19: A Randomized-Controlled Trial Jul 2020, Clinical Infectious Diseases, ciaa1009, Volume 73, Issue 11, Page e4073-e4081
EARLY TREATMENT 293 patient HCQ early treatment RCT: 16% lower hospitalization (p=0.64), 34% improved recovery (p=0.38), and 2% improved viral clearance.
This paper has conflicting values, table S2 shows 12 control hospitalizations, while table 2 shows 11. The original report for this paper had more conflicting values, with values reported in Table 2 and the abstract corresponding to 12 control hospitalizations, while others corresponded to 11 control hospitalizations. The counts in table S2 also do not match; n=290 is given for secondary endpoints but the three groups add up to n=238. The sum of the secondary endpoint counts for the control group in table 2 do not match the group size. One missing patient may be the 12th control hospitalization but there are 2 more missing. There was a 16% reduction in hospitalization and 34% reduction in the risk of no symptom resolution, without statistical significance due to small samples. Treatment delay is unknown. They report a delay of up to 120 hours after symptoms plus an additional unspecified delay where medication was provided to patients at the first home visit. Authors did not respond to C19early.com’s request for details. Authors do not break down results by treatment delay. The paper does not mention zinc. Zinc deficiency in Spain has been reported at 83%, this may significantly reduce effectiveness. HCQ is a zinc ionophore which increases cellular uptake, facilitating significant intracellular concentrations of zinc, and zinc is known to inhibit SARS-CoV RNA-dependent RNA polymerase activity, and is widely thought to be important for effectiveness of HCQ in SARS-CoV-2. Undetectable viral load was changed to 3 log10 copies/mL potentially altering effectiveness. For viral load authors use nasopharyngeal swabs, we note that viral activity in the lung may be especially important for COVID-19, and that research has shown HCQ concentrations can be much higher in the lung compared to plasma. We also note that viral detection by PCR does not equate to viable virus. Accuracy of the tests is not provided. Nasopharyngeal viral load analysis issues include test unreliability and temporo-spatial differences in viral shedding. 293 low-risk patients with no deaths. No serious adverse events. C19early.com attempted to correspond with authors, asking for more details on the treatment delay and viral load change but received no response. Also see this open letter. https://c19p.org/mitja
115. M. Chechter, G. Dutra da Silva, R. E Costa, T. Miklos, N. Antonio da Silva, G. Lorber, N. Vascncellos Mota, A. Dos Santos Cortada, L. De Nazare Lima da Cruz, P. De Melo, B. De Souza, F. Emmerich, P. De Andrade Zanotto, and M. Aaron Scheinberg, Evaluation of patients treated by telemedicine in the beginning of the COVID-19 pandemic in São Paulo, Brazil: A non-randomized clinical trial preliminary study Nov 2021, Heliyon, Page e15337
EARLY TREATMENT 72 patient HCQ early treatment study: 95% lower hospitalization (p=0.004).
Prospective study of 187 telemedicine patients in Brazil. 74 presenting with moderate symptoms were offered treatment with HCQ+AZ, 12 did not accept HCQ (taking AZ only), forming a control group. There was lower hospitalization and improved recovery with treatment. https://c19p.org/chechter
116. McCullough et al., Hydroxychloroquine in the Prevention of COVID-19 Infection in Healthcare Workers Aug 2021, NCT04333225
221 patient HCQ prophylaxis study: 52% fewer cases (p=0.01).
Prospective study with 221 healthcare workers, showing lower risk of COVID-19 with HCQ prophylaxis. https://c19p.org/mccullough4
117. M. Modrák, P. Bürkner, T. Sieger, T. Slisz, M. Vašáková, G. Mesežnikov, L. Casas-Mendez, J. Vajter, J. Táborský, V. Kubricht, D. Suk, J. Horejsek, M. Jedlička, A. Mifková, A. Jaroš, M. Kubiska, J. Váchalová, R. Šín, M. Veverková, Z. Pospíšil, J. Vohryzková, R. Pokrievková, K. Hrušák, K. Christozova, V. Leos-Barajas, K. Fišer, and T. Hyánek, Detailed disease progression of 213 patients hospitalized with Covid-19 in the Czech Republic: An exploratory analysis Dec 2020, medRxiv
LATE TREATMENT 213 patient HCQ late treatment study: 59% lower mortality (p=0.04).
Retrospective 213 hospitalized patients in Czech Republic showing lower mortality with HCQ. Subject to confounding by indication. https://c19p.org/modrak
118. A. Khurana, G. Kaushal, R. Gupta, V. Verma, K. Sharma, and M. Kohli, Prevalence and clinical correlates of COVID-19 outbreak among healthcare workers in a tertiary level hospital Jul 2020, medRxiv
181 patient HCQ prophylaxis study: 51% fewer cases (p=0.02).
Study of hospital health care workers showing HCQ prophylaxis reduces COVID-19 significantly, OR 0.30, p=0.02. 94 positive health care workers with a matched sample of 87 testing negative. Full course prophylaxis was important in this study which used a low dose of 400mg/week HCQ (800mg for week 1), so it may take longer to reach therapeutic levels. Actual benefit of HCQ may be larger because severity of symptoms are not considered here but HCQ may also reduce severity. https://c19p.org/khurana
119. F. Membrillo de Novales, G. Ramírez-Olivencia, M. Estébanez, B. De Dios, M. Herrero, T. Mata, A. Borobia, C. Gutiérrez, M. Simón, A. Ochoa, Y. Martínez, A. Aguirre, F. Alcántara, P. Fernández-González, E. López, S. Campos, M. Navarro, and L. Ballester, Early Hydroxychloroquine Is Associated with an Increase of Survival in COVID-19 Patients: An Observational Study May 2020, Preprints 2020, 2020050057
LATE TREATMENT 166 patient HCQ late treatment study: 55% lower mortality (p=0.002).
166 patients hospitalized with COVID-19, HCQ increased survival 1.4 – 1.8 times when patients admitted in early stages. Early is relative to hospital admission here – all patients were in relatively serious condition. https://c19p.org/membrillo
120. R. Rajasingham, A. Bangdiwala, M. Nicol, C. Skipper, K. Pastick, M. Axelrod, M. Pullen, A. Nascene, D. Williams, N. Engen, E. Okafor, B. Rini, I. Mayer, E. McDonald, T. Lee, P. Li, L. MacKenzie, J. Balko, S. Dunlop, K. Hullsiek, D. Boulware, S. Lofgren, M. Abassi, A. Balster, L. Collins, G. Drobot, D. Krakower, S. Lother, D. MacKay, C. Meyer-Mueller, S. Selinsky, D. Solvason, R. Zarychanski, and R. Zash, Hydroxychloroquine as pre-exposure prophylaxis for COVID-19 in healthcare workers: a randomized trial Sep 2020, Clinical Infectious Diseases, Volume 72, Issue 11, Page e835-e843
1,483 patient HCQ prophylaxis RCT: 27% fewer cases (p=0.07).
PrEP RCT showing lower cases with HCQ prophylaxis. The trial was halted after 47% enrollment, p < 0.05 would be reached at ~75% enrollment if similar results continued. HR 0.66/0.68 for full medication adherence, 0.72/0.74, p = 0.18/0.22 overall (1x/2x dosing). Efficacy for first responders was higher, OR 0.32, p = 0.01. First responders had a much higher incidence, allowing greater power, and reducing the effect of confounders such as misdiagnosis of other conditions or survey issues. Performance is similar to the control arm for the first 3 weeks. The effect may be greater with a dosage regimen that achieves therapeutic levels faster. ~40% of participants suspected they might have had COVID-19 before the trial, the effect in people without prior COVID-19 may be higher. Research shows the treatment used in the control arm (folic acid) may have significant efficacy for COVID-19, so the true effectiveness of HCQ may be higher than observed. Also see this article regarding foilc acid. Note that folic acid is predicted to bind to multiple SARS-CoV-2 proteins, folic acid levels are lower in COVID-19 patients with severe disease, folic acid supplementation may help with COVID-19 associated hypertension and hyperhomocystinemia, and differences in a folic acid-related enzyme could impact COVID-19 geographical severity variation. Authors note that the trial was underpowered, investigation into more frequent dosing is warranted, and that the dosing may have been insufficient with no participants achieving more than the in vitro EC50. Internet survey RCT subject to survey bias. There were no deaths or ICU admissions. Low risk healthcare workers, median age ~40. 494 1x/week dosing, 495 2x/week dosing, 494 control participants (1x and 2x participants received the same overall dosage). https://c19p.org/rajasingham
121. B. Singh, B. Moirangthem, P. Panda, Y. Bahurupi, S. Saha, G. Saini, M. Dhar, M. Bairwa, V. Pai, A. Agarwal, G. Sindhwani, S. Handu, and Ravikant, Safety and efficacy of antiviral therapy alone or in combination in COVID-19 – a randomized controlled trial (SEV COVID Trial) Jun 2021, medRxiv
LATE TREATMENT 74 patient HCQ late treatment RCT: 48% lower mortality (p=0.45) and 14% improved recovery (p=0.76).
Very small early terminated RCT in India, showing lower mortality but without statistical significance with the very small sample size. Time since symptom onset is not provided. The recovery percentage for non-severe group B (86.7%) does not match any number of recoveries, we have used the closest number (15/17). https://c19p.org/singh2
122. S. Almazrou, Z. Almalki, A. Alanazi, A. Alqahtani, and S. Alghamd, Comparing the impact of Hydroxychloroquine based regimens and standard treatment on COVID-19 patient outcomes: A retrospective cohort study Sep 2020, Saudi Pharmaceutical J., Volume 28, Issue 12, Page 1877-1882
LATE TREATMENT 161 patient HCQ late treatment study: 65% lower ventilation (p=0.16) and 21% lower ICU admission (p=0.78).
Retrospective 161 hospitalized patients in Saudi Arabia showing lower ventilation and ICU admission with HCQ, but not statistically significant with the small sample sizes. https://c19p.org/almazrou
123. C. Gentry, M. Humphrey, S. Thind, S. Hendrickson, G. Kurdgelashvili, and R. Williams, Long-term hydroxychloroquine use in patients with rheumatic conditions and development of SARS-CoV-2 infection: a retrospective cohort study Sep 2020, Lancet Rheumatology, Volume 2, Issue 11, Page e689-e697
32,109 patient HCQ prophylaxis study: 91% lower mortality (p=0.1) and 21% fewer cases (p=0.27).
Retrospective patients with rheumatologic conditions showing zero of 10,703 COVID-19 deaths for HCQ patients versus 7 of 21,406 propensity matched control patients (not statistically significant). The average age of HCQ patients is slightly lower 64.8 versus 65.4 control. COVID-19 cases OR 0.79, p=0.27. There are several significant differences in the propensity matched patients that could affect results, e.g., 20.9% SLE versus 24.7%. https://c19p.org/gentry
124. K. Said, A. Alsolami, F. Alreshidi, A. Fathuddin, F. Alshammari, F. Alrashid, A. Aljadani, R. Aboras, F. Alreshidi, M. Alghozwi, S. Alshammari, and N. Alharbi, Profiles of Independent-Comorbidity Groups in Senior COVID-19 Patients Reveal Low Fatality Associated with Standard Care and Low-Dose Hydroxychloroquine over Antivirals Apr 2023, J. Multidisciplinary Healthcare, Volume Volume 16, Page 1215-1229
LATE TREATMENT 840 patient HCQ late treatment study: 78% lower mortality (p<0.0001).
Retrospective 750 COVID-19 patients in Saudi Arabia, showing lower mortality with HCQ treatment in unadjusted results. Authors note that the poor results in some other trials may be related to increased dosages and later treatment. https://c19p.org/said2
125. E. Satti, M. Ostensen, S. Darrgham, N. Hadwan, H. Ashour, and S. AL Emadi, Characteristics and Obstetric Outcomes in Women With Autoimmune Rheumatic Disease During the COVID-19 Pandemic in Qatar Apr 2022, Cureus
80 patient HCQ prophylaxis study: 61% fewer cases (p=0.04).
Retrospective 80 consecutive pregnant patients with autoimmune rheumatic diseases in Qatar, showing lower risk of COVID-19 cases with HCQ prophylaxis. https://c19p.org/satti
126. M. AbdelGhaffar, D. Omran, A. Elgebaly, E. Bahbah, S. Afify, M. AlSoda, M. El-Shiekh, E. ElSayed, S. Shaaban, S. AbdelHafez, K. Elkelany, A. Eltayar, O. Ali, L. Kamal, A. Heiba, A. El Askary, and H. Shousha, Prediction of mortality in hospitalized Egyptian patients with Coronavirus disease-2019: A multicenter retrospective study Jan 2022, PLOS ONE, Volume 17, Issue 1, Page e0262348
LATE TREATMENT 3,712 patient HCQ late treatment study: 100% lower mortality (p<0.0001).
Retrospective 3,712 hospitalized patients in Egypt, showing lower mortality with HCQ treatment in unadjusted results. According to the official treatment protocol, HCQ was recommended with higher risk and/or more serious cases. https://c19p.org/abdelghaffar
127. Y. Huang, Z. Chen, Y. Wang, L. Han, K. Qin, W. Huang, Y. Huang, H. Wang, P. Shen, X. Ba, W. Lin, H. Dong, M. Zhang, and S. Tu, Clinical characteristics of 17 patients with COVID-19 and systemic autoimmune diseases: a retrospective study Jun 2020, Annals of the Rheumatic Diseases 2020:79, 1163-1169, Volume 79, Issue 9, Page 1163-1169
1,255 patient HCQ prophylaxis study: 80% lower hospitalization (p=0.001).
Analysis of 1255 COVID-19 patients in Wuhan Tongji Hospital finding 0.61% with systemic autoimmune diseases, much lower than authors expected (3%-10%). Authors hypothesise that protective factors, such as CQ/HCQ use, reduce hospitalization. https://c19p.org/huangard
128. K. Oku, Y. Kimoto, T. Horiuchi, M. Yamamoto, Y. Kondo, M. Okamoto, T. Atsumi, and T. Takeuchi, Risk factors for hospitalization or mortality for COVID-19 in patients with rheumatic diseases: Results of a nation-wide JCR COVID-19 registry in Japan Sep 2022, Modern Rheumatology
220 patient HCQ prophylaxis study: 92% lower mortality (p=1) and 12% lower hospitalization (p=0.34).
Retrospective 220 COVID-19 patients with rheumatic disease in Japan, showing lower mortality and hospitalization with HCQ prophylaxis, without statistical significance. https://c19p.org/oku
129. G. Ramírez-García, P. García-Molina, M. Flor-Cremades, B. Muñoz-Rojas, J. Moleón Moya, Hydroxychloroquine and Tocilizumab in the Treatment of COVID-19: A Longitudinal Observational Study May 2021, Archivos de Medicina Universitaria
LATE TREATMENT 403 patient HCQ late treatment study: 67% lower mortality (p<0.0001) and 6% higher ICU admission (p=1).
Retrospective 403 hospitalized patients in Spain, showing lower mortality with treatment, however authors do not adjust for the differences between the groups. Confounding by indication is likely. https://c19p.org/ramirezgarcia
130. G. Meeus, F. Van Coile, H. Pottel, A. Michel, O. Vergauwen, K. Verhelle, S. Lamote, M. Leys, M. Boudewijns, and P. Samaey, Efficacy and safety of in-hospital treatment of Covid-19 infection with low-dose hydroxychloroquine and azithromycin in hospitalized patients: A retrospective controlled cohort study Sep 2023, New Microbes and New Infections, Page 101172
LATE TREATMENT 3,885 patient HCQ late treatment study: 36% lower mortality (p=0.005).
Retrospective 352 hospitalized COVID-19 patients in Belgium and 3,533 control patients from the contemporaneous Belgian Collaborative Group, showing significantly lower mortality with HCQ treatment. The survival benefit was consistent in all age groups. No torsade de pointes or ventricular arrhythmias were observed. Mean time from onset is not provided, but 43% of patients with known onset were admitted within 5 days, making the efficacy consistent with expectations based on the treatment delay. HCQ 800mg day one, 200mg bid for five days, according to national guidelines. Authors note that the poor results in SOLIDARITY/RECOVERY trials may be related to the excessively high doses used. Most patients also received AZ. Adjusted results are only provided for all HCQ patients. Publication was delayed over 3 years. Authors reported in 2021 that the paper had been rejected by the editors of four different journals, prior to peer review. https://c19p.org/meeus
131. C. Johnston, E. Brown, J. Stewart, H. Karita, P. Kissinger, J. Dwyer, S. Hosek, T. Oyedele, M. Paasche-Orlow, K. Paolino, K. Heller, H. Leingang, H. Haugen, T. Dong, A. Bershteyn, A. Sridhar, J. Poole, P. Noseworthy, M. Ackerman, S. Morrison, A. Greninger, M. Huang, K. Jerome, M. Wener, A. Wald, J. Schiffer, C. Celum, H. Chu, R. Barnabas, and J. Baeten, Hydroxychloroquine with or Without Azithromycin for Treatment of Early SARS-CoV-2 Infection Among High-Risk Outpatient Adults: A Randomized Clinical Trial Dec 2020, EClinicalMedicine, Volume 33, Page 100773
LATE TREATMENT 231 patient HCQ late treatment RCT: 30% lower hospitalization (p=0.73), 2% improved recovery (p=0.95), and 29% faster viral clearance.
Small early terminated late treatment RCT comparing vitamin C + folic acid, HCQ + folic acid, and HCQ+AZ, showing non-statistically significantly lower hospitalization with HCQ/HCQ+AZ, and faster viral clearance with HCQ. Enrollment was a median of 5.9 days after onset (6.2 and 6.3 in the treatment arms). The median time to viral clearance for vitamin C + folic acid was 8 days in the preprint but changed to 7 days in the published paper without explanation. Both vitamin C and folic acid (here and here) show efficacy in other trials, so the true effectiveness of HCQ(+AZ) may be higher than observed. Low risk patients, median age 37, no deaths (not matching the title which claims “high risk”). Post hoc addition of a new cycle threshold to obscure the statistically significant faster clearance. No analysis for time from symptom onset. Authors identify (relatively) low and high risk cohorts, but do not provide either viral shedding or symptom resolution results for the cohorts. NCT04354428. https://c19p.org/johnston
132. A. Alshamrani, A. Assiri, and O. Almohammed, Comprehensive evaluation of six interventions for hospitalized patients with COVID-19: A propensity score matching study Feb 2023, Saudi Pharmaceutical J.
LATE TREATMENT 814 patient HCQ late treatment PSM study: 50% lower mortality (p=0.18), 37% lower progression (p=0.21), 9% shorter ICU admission (p=0.66), and 3% longer hospitalization (p=0.7).
PSM retrospective 29 hospitals in Saudi Arabia, finding lower mortality with HCQ, without reaching statistical significance (described by authors as “no impact”). https://c19p.org/alshamrani
133. Á. Avezum, G. Oliveira, H. Oliveira, R. Lucchetta, V. Pereira, A. Dabarian, R. D´O Vieira, D. Silva, A. Kormann, A. Tognon, R. De Gasperi, M. Hernandes, A. Feitosa, A. Piscopo, A. Souza, C. Miguel, V. Nogueira, C. Minelli, C. Magalhães, K. Morejon, L. Bicudo, G. Souza, M. Gomes, J. Fo, A. Schwarzbold, A. Zilli, R. Amazonas, F. Moreira, L. Alves, S. Assis, P. Neves, J. Matuoka, I. Boszczowski, D. Catarino, V. Veiga, L. Azevedo, R. Rosa, R. Lopes, A. Cavalcanti, and O. Berwanger, Hydroxychloroquine versus placebo in the treatment of non-hospitalised patients with COVID-19 (COPE – Coalition V): A double-blind, multicentre, randomised, controlled trial Mar 2022, The Lancet Regional Health – Americas, Volume 11, Page 100243
EARLY TREATMENT 1,372 patient HCQ early treatment RCT: 1% lower mortality (p=1), 32% higher ventilation (p=0.79), 16% lower ICU admission (p=0.61), and 23% lower hospitalization (p=0.18).
Authors have not responded to a request for source data form C19early.com. Outpatient RCT with 687 HCQ and 682 control patients in Brazil, showing lower hospitalization with treatment, not reaching statistical significance. Higher efficacy was seen with treatment <4 days from onset, RR 0.61. The associated meta analysis includes mostly late treatment studies, for example in the median delay from onset was 7 days is missing. The values for are incorrect – the study shows 4 hospitalizations in the control arm – RR for this study should be 0.58 instead of 0.78. https://c19p.org/avezum
134. A. Delgado, B. Cornett, Y. Choi, C. Colosimo, V. Stahel, O. Dziadkowiec, and P. Stahel, Investigational medications in 9,638 hospitalized patients with severe COVID-19: lessons from the “fail-and-learn” strategy during the first two waves of the pandemic in 2020 Feb 2023, Research Square
LATE TREATMENT 9,638 patient HCQ late treatment study: 26% lower mortality (p=0.002).
PSM retrospective 9,638 patients in the USA, showing significantly lower mortality with HCQ in early 2020 (1,157 HCQ patients), and no significant difference in late 2020 (82 HCQ patients). The few patients treated in the later period may be in more serious condition due to the effort required to overcome the politicization and censorship in the study country. Authors refer to their result as “no relevant benefit in mortality between the two surges.” https://c19p.org/delgado
135. A. AlShehhi, T. Almansoori, A. Alsuwaidi, and H. Alblooshi, Utilizing machine learning for survival analysis to identify risk factors for COVID-19 intensive care unit admission: A retrospective cohort study from the United Arab Emirates Jan 2024, PLOS ONE, Volume 19, Issue 1, Page e0291373
LATE TREATMENT 1,797 patient HCQ late treatment study: 43% lower ICU admission (p=0.001).
Retrospective 1,787 hospitalized COVID-19 patients in the United Arab Emirates, identifying hydroxychloroquine as reducing the risk of ICU admission in a machine learning model. Only unadjusted quantitative results are provided, which also show a benefit. https://c19p.org/alshehhi
136. M. Sahebari, Z. Mirfeizi, Z. Shariati-Sarabi, M. Moghadam, K. Hashemzadeh, and M. Firoozabadi, Influence of biologic and conventional disease-modifying antirheumatic drugs on COVID-19 incidence among rheumatic patients during the first and second wave of the pandemic in Iran Sep 2022, Reumatologia/Rheumatology, Volume 60, Issue 4, Page 231-241
512 patient HCQ prophylaxis study: 56% fewer cases (p=0.02).
Retrospective 512 rheumatic disease patients in Iran, showing lower risk of COVID-19 with HCQ use. https://c19p.org/sahebari
137. D. MacFadden, K. Brown, S. Buchan, H. Chung, R. Kozak, J. Kwong, D. Manuel, S. Mubareka, and N. Daneman, Screening Large Population Health Databases for Potential COVID-19 Therapeutics: A Pharmacopeia-Wide Association Study (PWAS) of Commonly Prescribed Medications Mar 2022, Open Forum Infectious Diseases
HCQ prophylaxis study: 12% fewer cases (p=0.01).
Retrospective 26,121 cases and 2,369,020 controls ≥65yo in Canada, showing lower cases with chronic use of HCQ. https://c19p.org/macfadden
138. A. Ahmed, W. Alotaibi, M. Aldubayan, A. Alhowail, A. Al-Najjar, S. Chigurupati, and R. Elgharabawy, Factors Affecting the Incidence, Progression, and Severity of COVID-19 in Type 1 Diabetes Mellitus Nov 2021, BioMed Research Int., Volume 2021, Page 1-9
100 patient HCQ prophylaxis study: 99% fewer cases (p=0.08).
Retrospective type 1 diabetes patients in Saudi Arabia showing reduced risk of cases with HCQ prophylaxis. https://c19p.org/ahmed2
139. K. Shaw, L. Yin, J. Shah, R. Sally, K. Svigos, P. Adotama, H. Tuan, J. Shapiro, R. Betensky, and K. Lo Siccoa, COVID-19 in Individuals Treated With Long-Term Hydroxychloroquine: A Propensity Score-Matched Analysis of Cicatricial Alopecia Patients Jun 2021, J. Drugs in Dermatology, Volume 20, Issue 8, Page 914-916
144 patient HCQ prophylaxis PSM study: 13% fewer cases (p=0.006).
PSM retrospective 144 alopecia patients in the USA, showing lower risk of COVID-19 with HCQ prophylaxis. The supplemental appendix is not available. https://c19p.org/shaw
140. M. Barry, N. Althabit, L. Akkielah, A. AlMohaya, M. Alotaibi, S. Alhasani, A. Aldrees, A. AlRajhi, A. AlHiji, F. Almajid, A. AlSharidi, F. Al-Shahrani, N. Alotaibi, and A. AlHetheel, Clinical Characteristics and Outcomes of Hospitalized COVID-19 Patients in a MERS-CoV Referral Hospital during the Peak of the Pandemic Mar 2021, Int. J. Infectious Diseases, Volume 106, Page 43-51
LATE TREATMENT 605 patient HCQ late treatment study: 99% lower mortality (p=0.6).
605 hospitalized patients in Saudi Arabia showing no mortality with HCQ (only 6 patients received HCQ). https://c19p.org/barry
141. R. Guner, I. Hasanoglu, B. Kayaaslan, A. Aypak, E. Akinci, H. Bodur, F. Eser, A. Kaya Kalem, O. Kucuksahin, I. Ates, A. Bastug, Y. Tezer Tekce, Z. Bilgic, F. Gursoy, H. Akca, S. Izdes, D. Erdem, E. Asfuroglu, H. Hezer, H. Kilic, M. Cıvak, S. Aydogan, and T. Buzgan, Comparing ICU Admission Rates of Mild/Moderate COVID-19 Patients Treated with Hydroxychloroquine, Favipiravir, and Hydroxychloroquine plus Favipiravir Dec 2020, J. Infection and Public Health, Volume 14, Issue 3, Page 365-370
LATE TREATMENT 704 patient HCQ late treatment study: 77% lower ICU admission (p=0.16).
Retrospective 824 hospitalized patients in Turkey showing lower ICU admission for HCQ vs. favipiravir. https://c19p.org/guner
142. M. Falcone, G. Tiseo, G. Barbieri, V. Galfo, A. Russo, A. Virdis, F. Forfori, F. Corradi, F. Guarracino, L. Carrozzi, A. Celi, M. Santini, F. Monzani, S. De Marco, M. Pistello, R. Danesi, L. Ghiadoni, A. Farcomeni, F. Menichetti, A. Sabrina, A. Rachele, B. Rubia, B. Pietro, B. Martina, B. Matteo, B. Giulia, C. Valeria, C. Nicoletta, C. Francesco, C. Alessandro, D. Alessandra, D. Massimiliano, F. Giovanna, G. Marco, M. Fabrizio, M. Alessandro, M. Paolo, M. Stefano, M. Marco, M. Alessandra, N. Elia, P. Naria, P. Simone, P. Chiara, R. Francesca, S. Maria, S. Massimiliano, and S. Stefano, Role of low-molecular weight heparin in hospitalized patients with SARS-CoV-2 pneumonia: a prospective observational study Nov 2020, Open Forum Infectious Diseases, Volume 7, Issue 12
LATE TREATMENT 315 patient HCQ late treatment PSM study: 65% lower mortality (p=0.2).
Prospective observational study of 315 hospitalized patients in Italy showing 65% lower mortality with HCQ. The median treatment delay was 6 days for survivors and 6.5 days for non-survivors. Mortality relative risk: RR 0.35, p = 0.2, propensity score matched RR 0.75, p = 0.36, multivariate Cox regression RR 0.43, p < 0.001, univariate Cox regression https://c19p.org/falcone
143. G. Boari, G. Chiarini, S. Bonetti, P. Malerba, G. Bianco, C. Faustini, F. Braglia-Orlandini, D. Turini, V. Guarinoni, M. Saottini, S. Viola, G. Ferrari-Toninelli, G. Pasini, C. Mascadri, B. Bonzi, P. Desenzani, C. Tusi, E. Zanotti, M. Nardin, and D. Rizzoni, Prognostic factors and predictors of outcome in patients with COVID-19 and related pneumonia: a retrospective cohort study Nov 2020, Biosci. Rep., Volume 40, Issue 12
LATE TREATMENT 258 patient HCQ late treatment study: 55% lower mortality (p=0.001).
Retrospective 258 hospitalized patients in Italy showing lower mortality with HCQ treatment, unadjusted relative risk RR 0.455, p<0.001. Data is in the supplementary appendix. https://c19p.org/boari
144. D. Águila-Gordo, J. Martínez-del Río, V. Mazoteras-Muñoz, M. Negreira-Caamaño, P. Nieto-Sandoval Martín de la Sierra, and J. Piqueras-Flores, Mortality and associated prognostic factors in elderly and very elderly hospitalized patients with respiratory disease COVID-19 Nov 2020, Revista Española de Geriatría y Gerontología, Volume 56, Issue 5, Page 259-267
LATE TREATMENT 416 patient HCQ late treatment study: 67% lower mortality (p=0.1).
67% lower mortality with HCQ. Retrospective 416 elderly patients in Spain showing adjusted HCQ mortality hazard ratio HR 0.33, p = 0.1. https://c19p.org/aguilagordo
145. E. Coll, M. Fernández-Ruiz, J. Sánchez-Álvarez, J. Martínez-Fernández, M. Crespo, J. Gayoso, T. Bada-Bosch, F. Oppenheimer, F. Moreso, M. López-Oliva, E. Melilli, M. Rodríguez-Ferrero, C. Bravo, E. Burgos, C. Facundo, I. Lorenzo, Í. Yañez, C. Galeano, A. Roca, M. Cabello, M. Gómez-Bueno, M. García-Cosío, J. Graus, L. Lladó, A. De Pablo, C. Loinaz, B. Aguado, D. Hernández, and B. Domínguez-Gil, Covid-19 in transplant recipients: the spanish experience Oct 2020, American J. Transplantation, Volume 21, Issue 5, Page 1825-1837
LATE TREATMENT 635 patient HCQ late treatment study: 46% lower mortality (p<0.0001).
Retrospective 652 transplant recipient patients in Spain showing 46% lower mortality for patients treated with HCQ, unadjusted relative risk RR 0.54, p<0.0001. https://c19p.org/coll
146. B. Grau-Pujol, D. Camprubí-Ferrer, H. Marti-Soler, M. Fernández-Pardos, C. Carreras-Abad, M. Andrés, E. Ferrer, M. Muelas-Fernandez, S. Jullien, G. Barilaro, S. Ajanovic, I. Vera, L. Moreno, E. Gonzalez-Redondo, N. Cortes-Serra, M. Roldán, A. Arcos, I. Mur, P. Domingo, F. Garcia, C. Guinovart, and J. Muñoz, Pre-exposure prophylaxis with hydroxychloroquine for COVID-19: a double-blind, placebo-controlled randomized clinical trial Sep 2020, Trials, Volume 22, Issue 1
269 patient HCQ prophylaxis RCT: 11% fewer cases (p=1).
Small PrEP RCT showing that PrEP with HCQ is safe at the dosage used. There were no deaths, hospitalizations, or serious adverse events. The paper states: “Among all trial participants at the end of the first month (n=253), only one participant from the placebo arm (1/116, 0.8%), tested positive for SARS-CoV-2 PCR and for a SARS-CoV-2 serology test.” The abstract states: “only one participant in each group was diagnosed with COVID-19.” https://c19p.org/graupujol
147. J. Berenguer, P. Ryan, J. Rodríguez-Baño, I. Jarrín, J. Carratalà, J. Pachón, M. Yllescas, J. Arriba, E. Aznar Muñoz, P. Gil Divasson, P. González Muñiz, C. Muñoz Aguirre, J. López, M. Ramírez-Schacke, I. Gutiérrez, F. Tejerina, T. Aldámiz-Echevarría, C. Díez, C. Fanciulli, L. Pérez-Latorre, F. Parras, P. Catalán, M. García-Leoni, I. Pérez-Tamayo, L. Puente, J. Cedeño, J. Berenguer, M. Díaz Menéndez, F. De la Calle Prieto, M. Arsuaga Vicente, E. Trigo Esteban, M. Lago Núñez, R. De Miguel Buckley, J. Cadiñaños Loidi, C. Busca Arenzana, A. Mican, M. Mora Rillo, J. Ramos Ramos, B. Loeches Yagüe, J. Bernardino de la Serna, J. García Rodríguez, J. Arribas López, A. Such Diaz, E. Álvaro Alonso, E. Izquierdo García, J. Torres Macho, G. Cuevas Tascon, J. Troya García, B. Mestre Gómez, E. Jiménez González de Buitrago et al., Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain Aug 2020, Clinical Microbiology and Infection, Volume 26, Issue 11, Page 1525-1536
LATE TREATMENT 3,995 patient HCQ late treatment study: 18% lower mortality (p=0.0001).
Retrospective 4035 hospitalized patients in Spain showing reduced mortality with HCQ (data is in the supplementary appendix). https://c19p.org/berenguer
148. K. Faíco-Filho, D. Conte, L. De Souza Luna, J. Carvalho, A. Perosa, and N. Bellei, No benefit of hydroxychloroquine on SARS-CoV-2 viral load reduction in non-critical hospitalized patients with COVID-19 Jun 2020, Braz J Microbiol, Volume 51, Issue 4, Page 1765-1769
LATE TREATMENT 66 patient HCQ late treatment study: 81% improved viral reduction rate (p=0.4).
Viral load comparison for 34 HCQ and 32 control patients hospitalized with moderate COVID-19. All patients recovered limiting the room for beneficial effects. While not achieving statistical significance, results show faster recovery with HCQ. The greatest benefit is seen mid-recovery as expected for an effective treatment: Δt7-12: 81% improvement with HCQ Δt<7: 24% improvement with HCQ For Δt>12, everyone has recovered so there is no room for improvement. Since the HCQ group started slightly higher the improvement is slightly less. Most participants have also dropped out by this test, with only 6 HCQ and 9 control remaining (also suggesting HCQ patients recovered faster). https://c19p.org/faicofilho
149. J. Mathew, S. Jain, T. Susngi, S. Naidu, V. Dhir, A. Sharma, S. Jain, and S. Sharma, Predictors of COVID-19 severity and outcomes in Indian patients with rheumatic diseases: a prospective cohort study Feb 2023, Rheumatology Advances in Practice, Volume 7, Issue 1
64 patient HCQ prophylaxis study: 20% lower mortality (p=0.8), no change in hospitalization (p=0.94), and 40% lower severe cases (p=0.37).
Prospective study of 64 rheumatic disease patients with COVID-19, showing no significant difference in outcomes with HCQ use. https://c19p.org/mathew
150. R. AlSulaiman, S. Alqatari, A. Nemer, M. Hasan, R. Bukhari, R. Al Argan, D. Al Khafaji, A. Alwaheed, A. Alzaki, M. Al-wazza, S. Al Warthan, A. Al Saeed, F. Albeladi, H. Almeer, and A. Abu Quren, COVID-19 in patients with rheumatological diseases in the Eastern Province of Saudi Arabia May 2023, J. Medicine and Life, Volume 16, Issue 6, Page 873-882
34 patient HCQ prophylaxis study: 89% lower ventilation (p=0.13), 64% lower ICU admission (p=0.14), and 64% lower severe cases (p=0.14).
Retrospective 34 rheumatological disease patients with COVID-19 in Saudi Arabia, showing lower risk of severe cases with HCQ use in unadjusted results, without statistical significance. https://c19p.org/alqatari
151. V. Raabe, A. Fleming, M. Samanovic, L. Lai, H. Belli, M. Mulligan, and H. Belmont, Hydroxychloroquine pre-exposure prophylaxis to prevent SARS-CoV-2 among health care workers at risk for SARS-CoV-2 exposure: A nonrandomized controlled trial Jul 2022, medRxiv
130 patient HCQ prophylaxis study: 82% fewer symptomatic cases (p=0.17).
Small prophylaxis study with 130 healthcare workers in the USA, showing lower symptomatic cases with HCQ prophylaxis, without statistical significance. HCQ participants were significantly older. The only symptomatic HCQ patient reported headache only as a potential COVID-19 symptom. https://c19p.org/raabe
152. N. Sawanpanyalert, R. Sirijatuphat, P. Sangsayunh, O. Putcharoen, W. Manosuthi, P. Intalapaporn, N. Palavutitotai, W. Samritmanoporn, N. Jitrungruengnij, A. Maleesatharn, K. Chokephaibulkit, Assessment of outcomes following implementation of antiviral treatment guidelines for COVID-19 during the first wave in Thailand Sep 2021, Southeast Asian J. Tropical Medicine and Public Health
EARLY TREATMENT HCQ early treatment study: 42% lower progression (p=0.37).
Retrospective 744 hospitalized patients in Thailand, showing lower risk of a poor outcome for favipiravir treatment within 4 days of symptom onset. Early treatment with CQ/HCQ and lopinavir/ritonavir or darunavir/ritonavir also showed lower risk, but without statistical significance. Sample sizes for the number of patients treated within 4 days of symptom onset are not provided. https://c19p.org/sawanpanyalert
153. B. Adama, P. Armel, C. Kadari, S. Apoline K, O. Boukary, O. Abdoul Risgou, T. Alfred B, K. Pierre, B. Brice W, Z. Jacques, S. Adama, F. Souleymane, K. Flavien, S. Adama, and K. Séni, Effect of Hydroxychloroquine or Chloroquine and Azithromycin on COVID-19 Patients’ Recovery and Mortality: Evidence from a Hospital Based Retrospective Cohort Study Conducted in Burkina Faso Feb 2021, J. Infectious Diseases and Epidemiology, Volume 7, Issue 2
LATE TREATMENT 208 patient HCQ late treatment study: 44% lower mortality (p=0.14) and 3% improved recovery (p=0.91).
Retrospective 208 hospitalized COVID-19 patients in Burkina Faso showing lower mortality with HCQ/CQ+AZ treatment, without statistical significance. There was no difference for recovery. https://c19p.org/baguiya
154. G. Lano, A. Braconnier, S. Bataille, G. Cavaille, J. Moussi-Frances, B. Gondouin, P. Bindi, M. Nakhla, J. Mansour, P. Halin, B. Levy, E. Canivet, K. Gaha, I. Kazes, N. Noel, A. Wynckel, A. Debrumetz, N. Jourde-Chiche, V. Moal, R. Vial, V. Scarfoglière, M. Bobot, M. Gully, T. Legris, M. Pelletier, M. Sallee, S. Burtey, P. Brunet, T. Robert, and P. Rieu, Risk factors for severity of COVID-19 in chronic dialysis patients from a multicentre French cohort Oct 2020, Clinical Kidney J., October 2020, 878–888, Volume 13, Issue 5, Page 878-888
LATE TREATMENT 122 patient HCQ late treatment study: 33% lower mortality (p=0.28) and 39% lower combined mortality/ICU admission (p=0.23).
33% lower mortality with HCQ+AZ, p=0.28. Retrospective 122 French dialysis patients. 69% lower combined mortality/ICU, p=0.11, for the subgroup not requiring O2 on diagnosis (slightly earlier treatment). https://c19p.org/lano
155. J. Nachega, D. Ishoso, J. Otokoye, M. Hermans, R. Machekano, N. Sam-Agudu, C. Bongo-Pasi Nswe, P. Mbala-Kingebeni, J. Madinga, S. Mukendi, M. Kolié, E. Nkwembe, G. Mbuyi, J. Nsio, D. Mukeba Tshialala, M. Tshiasuma Pipo, S. Ahuka-Mundeke, J. Muyembe-Tamfum, L. Mofenson, G. Smith, E. Mills, J. Mellors, A. Zumla, D. Mavungu Landu, and J. Kayembe, Clinical Characteristics and Outcomes of Patients Hospitalized for COVID-19 in Africa: Early Insights from the Democratic Republic of the Congo Oct 2020, The American J. Tropical Medicine and Hygiene, Volume 103, Issue 6, Page 2419-2428
LATE TREATMENT 766 patient HCQ late treatment study: 28% lower mortality (p=0.17) and 26% greater improvement (p=0.13).
Retrospective 766 hospitalized patients in DRC showing mortality reduced from 29% to 11%, and improvement at 30 days increased from 65% to 84%. Mortality cox regression adjusted hazard ratio aHR 0.26, p < 0.001 Risk of no improvement adjusted odds ratio 0.28, p < 0.001 Using marginal structural model analysis these risks became: Mortality MSM adjusted odds ratio adjusted odds ratio 0.65, p = 0.166. Risk of no improvement MSM adjusted odds ratio adjusted odds ratio = 0.65, p = 0.132 Median age 46, 630 treated with CQ+AZ. https://c19p.org/nachega
156. B. Kirenga, W. Muttamba, A. Kayongo, C. Nsereko, T. Siddharthan, J. Lusiba, L. Mugenyi, R. Byanyima, W. Worodria, F. Nakwagala, R. Nantanda, I. Kimuli, W. Katagira, B. Bagaya, E. Nasinghe, H. Aanyu-Tukamuhebwa, B. Amuge, R. Sekibira, E. Buregyeya, N. Kiwanuka, M. Muwanga, S. Kalungi, M. Joloba, D. Kateete, B. Byarugaba, M. Kamya, H. Mwebesa, and W. Bazeyo, Characteristics and outcomes of admitted patients infected with SARS-CoV-2 in Uganda Sep 2020, BMJ Open Respiratory Research, Volume 7, Issue 1, Page e000646
56 patient HCQ early treatment study: 26% faster recovery (p=0.2).
Prospective 56 patients in Uganda, 29 HCQ and 27 control, showing 25.6% faster recovery with HCQ, 6.4 vs. 8.6 days (p = 0.20). There was no ICU admission, mechanical ventilation, or death. Treatment delay is not specified but at least a portion of patients appear to have been treated early. https://c19p.org/kirenga
157. P. Byakika-Kibwika, C. Sekaggya-Wiltshire, J. Semakula, J. Nakibuuka, J. Musaazi, J. Kayima, C. Sendagire, D. Meya, B. Kirenga, S. Nanzigu, A. Kwizera, F. Nakwagala, I. Kisuule, M. Wayengera, H. Mwebesa, M. Kamya, and W. Bazeyo, Safety and Efficacy of Hydroxychloroquine for Treatment of Non-Severe COVID-19 in Adults in Uganda: A Randomized Open Label Phase II Clinical Trial Jun 2021, Research Square
LATE TREATMENT 105 patient HCQ late treatment RCT: no change in recovery (p=0.91) and 29% improved viral clearance (p=0.47).
Small 105 patient RCT in Uganda showing no significant differences. No mortality was reported. The patients were very young (median age 32), recovering in a median time of 3 days with standard of care, leaving little room for a treatment to make improvements. Time since symptom onset is not specified, but the distribution of symptoms at baseline suggests that the enrollment is relatively late within a cohort of low risk patients. https://c19p.org/byakikakibwika
158. S. Budhiraja, A. Soni, V. Jha, A. Indrayan, A. Dewan, O. Singh, Y. Singh, I. Chugh, V. Arora, R. Pande, A. Ansari, and S. Jha, Clinical Profile of First 1000 COVID-19 Cases Admitted at Tertiary Care Hospitals and the Correlates of their Mortality: An Indian Experience Nov 2020, medRxiv
LATE TREATMENT 976 patient HCQ late treatment study: 65% lower mortality (p<0.0001).
Retrospective 976 hospitalized patients with 834 treated with HCQ+AZ showing HCQ mortality relative risk RR 0.35, p < 0.0001. Note that in this case HCQ was recommended for mild/moderate cases, so more severe cases may not have received HCQ (which may also be why they became severe cases). We note that this is opposite to a common bias in HCQ studies – in many cases HCQ was more likely to be given to more severe cases. https://c19p.org/budhiraja
159. Á. Aparisi, C. Iglesias-Echeverría, C. Ybarra-Falcón, I. Cusácovich, A. Uribarri, M. García-Gómez, R. Ladrón, R. Fuertes, J. Candela, W. Hinojosa, C. Dueñas, R. González, L. Nogales, D. Calvo, M. Carrasco-Moraleja, J. Román, I. Amat-Santos, and D. Andaluz-Ojeda, Low-density lipoprotein cholesterol levels are associated with poor clinical outcomes in COVID-19 Oct 2020, medRxiv
LATE TREATMENT 654 patient HCQ late treatment study: 63% lower mortality (p=0.008).
Retrospective 654 hospitalized patients focused on lower serum cholesterol levels, also showing results for HCQ with 605 HCQ patients, unadjusted 30 day mortality relative risk RR 0.37, p = 0.008. https://c19p.org/aparisi
160. Belmont et al., COVID-19 PrEP HCW HCQ Study Oct 2021, ClinicalTrials.gov, NCT04354870
80 patient HCQ prophylaxis study: 79% fewer symptomatic cases (p=0.21).
Prospective study of HCQ prophylaxis in the USA, with 56 HCQ patients and 24 control patients, showing no significant differences. NCT04354870 https://c19p.org/belmont
161. M. Agarwal, R. Ranka, P. Panda, A. Kumar, G. Chikara, S. Sharma, R. Negi, R. Samanta, R. Walia, Y. Bahurupi, S. Saha, M. Dhar, P. Sharma, A. Gupta, U. Mishra, M. Gupta, and R. Kant, Low dose hydroxychloroquine prophylaxis for COVID-19 – a prospective study Sep 2021, medRxiv
484 patient HCQ prophylaxis study: 27% lower progression (p=0.21) and 5% more cases (p=0.81).
Small prophylaxis trial with 29 low dose HCQ and 455 control healthcare workers in India, showing no statistically significant differences. https://c19p.org/agarwal2
162. C. Scirocco, S. Ferrigno, L. Andreoli, M. Fredi, C. Lomater, L. Moroni, M. Mosca, B. Raffeiner, G. Carrara, G. Landolfi, D. Rozza, A. Zanetti, C. Scirè, and G. Sebastiani, COVID-19 prognosis in systemic lupus erythematosus compared with rheumatoid arthritis and spondyloarthritis: results from the CONTROL-19 Study by the Italian Society for Rheumatology Oct 2023, Lupus Science & Medicine, Volume 10, Issue 2, Page e000945
627 patient HCQ prophylaxis study: 41% lower combined mortality/intubation (p=0.38).
Retrospective 103 SLE and 524 RA patients in Italy, showing significantly lower mortality/ventilation with HCQ use for SLE patients, and no significant difference for RA patients in unadjusted results. Authors did not include HCQ in multivariable analysis, only including four variables “chosen among the most clinically relevant.” Multivariable analysis may significantly improve results for RA patients because HCQ use may correlate with more severe disease due to use for patients that failed or do not tolerate first-line therapies. It is not clear how the patients were selected – the very high ~25% ventilation/mortality suggests that most were hospitalized COVID-19 patients, in which case any benefit of HCQ in reducing hospitalizations will not be reflected in the results. Authors falsely state that “subsequent studies have definitely proved that [HCQ] is not linked to COVID-19 prognosis”, suggesting significant bias, and possibly indicating why HCQ was excluded in the reported multivariable results. While such a negative statement is reasonable based on the evidence for very late stage high dose treatment, studies for early treatment and prophylaxis do not match. In reality, % of all studies show a positive effect, and % of early treatment and % of prophylaxis studies show a positive effect. Controlled studies show statistically significant positive results for one or more outcomes (including RCTs). https://c19p.org/scirocco
163. P. Sen, N. R, A. Nune, J. Day, M. Joshi, V. Agarwal, R. Aggarwal, and L. Gupta, Post-COVID-19 condition in patients with autoimmune rheumatic diseases: the COVID-19 Vaccination in Autoimmune Diseases (COVAD) study Apr 2023, The Lancet Rheumatology, Volume 5, Issue 5, Page e247-e250
HCQ long COVID study: 40% lower PASC (p=0.08).
Retrospective 755 autoimmune rheumatic disease patients, showing lower risk of PASC (long COVID) with HCQ use, without statistical significance. https://c19p.org/sen2
164. A. Krishnan, R. Kumar, R. Amarchand, A. Mohan, R. Kant, A. Agarwal, P. Kulshreshtha, P. Panda, A. Bhadoria, N. Agarwal, B. Biswas, R. Nair, N. Wig, R. Malhotra, S. Bhatnagar, R. Aggarwal, K. Soni, N. Madan, A. Trikha, P. Tiwari, A. Singh, M. Wyawahare, V. Gunasekaran, D. Sekar, S. Misra, P. Bhardwaj, A. Goel, N. Dutt, D. Kumar, N. Nagarkar, A. Galhotra, A. Jindal, U. Raj, A. Behera, S. Siddiqui, A. Kokane, R. Joshi, A. Pakhare, F. Farooque, S. Pawan, P. Deshmukh, R. Solanki, B. Rathod, V. Dutta, P. Mohapatra, M. Panigrahi, S. Barik, and R. Guleria, Predictors of Mortality among Patients Hospitalized with COVID-19 during the First Wave in India: A Multisite Case-Control Study Apr 2023, The American J. Tropical Medicine and Hygiene, Volume 108, Issue 4, Page 727-733
LATE TREATMENT 2,431 patient HCQ late treatment study: 40% lower mortality (p=0.05).
Case control study with 2,431 hospitalized COVID-19 patients in India, showing lower mortality with HCQ treatment, without statistical significance. https://c19p.org/krishnan2
165. A. Aweimer, L. Petschulat, B. Jettkant, R. Köditz, J. Finkeldei, J. Dietrich, T. Breuer, C. Draese, U. Frey, T. Rahmel, M. Adamzik, D. Buchwald, D. Useini, T. Brechmann, I. Hosbach, J. Bünger, A. Ewers, I. El-Battrawy, and A. Mügge, Mortality rates of severe COVID-19-related respiratory failure with and without extracorporeal membrane oxygenation in the Middle Ruhr Region of Germany Mar 2023, Scientific Reports, Volume 13, Issue 1
LATE TREATMENT 149 patient HCQ ICU study: 40% lower mortality (p=0.12).
Retrospective 149 patients under invasive mechanical ventilation in Germany showing no significant difference in mortality with HCQ in unadjusted results. https://c19p.org/aweimerh
166. K. Chevalier, M. Genin, T. Jean, J. Avouac, R. Flipo, S. Georgin-Lavialle, S. El Mahou, E. Pertuiset, T. Pham, A. Servettaz, H. Marotte, F. Domont, P. Chazerain, M. Devaux, A. Mekinian, J. Sellam, B. Fautrel, D. Rouzaud, E. Ebstein, N. Costedoat-Chalumeau, C. Richez, E. Hachulla, X. Mariette, and R. Seror, CovAID: Identification of factors associated with severe COVID-19 in patients with inflammatory rheumatism or autoimmune diseases Mar 2023, Frontiers in Medicine, Volume 10
1,213 patient HCQ prophylaxis study: 35% lower mortality (p=0.19) and 19% lower hospitalization (p=0.36).
Retrospective 1,213 rheumatic disease patients in France, showing lower risk of mortality and severe cases with HCQ use in univariate analysis, without statistical significance. https://c19p.org/chevalier
167. M. Opdam, S. Benoy, L. Verhoef, S. Van Bijnen, F. Lamers‐Karnebeek, R. Traksel, P. Vos, A. Den Broeder, and J. Broen, Identification of Risk Factors for COVID-19 Hospitalization in Patients with Anti-Rheumatic Drugs: Results from a Multicenter Nested Case Control Study Feb 2022, Clinical Pharmacology & Therapeutics
477 patient HCQ prophylaxis study: 45% lower hospitalization (p=0.18).
Retrospective 81 cases and 396 controls among rheumatic disease patients in the Netherlands, showing lower risk of hospitalization with HCQ prophylaxis, without statistical significance. https://c19p.org/opdam
168. R. Cordtz, S. Kristensen, L. Dalgaard, R. Westermann, K. Duch, J. Lindhardsen, C. Torp-Pedersen, and L. Dreyer, Incidence of COVID-19 Hospitalisation in Patients with Systemic Lupus Erythematosus: A Nationwide Cohort Study from Denmark Aug 2021, J. Clinical Medicine, Volume 10, Issue 17, Page 3842
2,533 patient HCQ prophylaxis study: 40% lower hospitalization (p=0.39).
Retrospective 2,533 SLE patients in Denmark showing no significant difference in hospitalization risk for COVID-19 cases with HCQ treatment. https://c19p.org/cordtz2
169. Q. Li, C. Cui, F. Xu, J. Zhao, N. Li, H. Li, T. Wang, H. Zhang, N. Liu, Y. Wei, X. Niu, Y. Xu, J. Dong, X. Yao, X. Wang, Y. Chen, H. Li, C. Song, J. Qiao, D. Liu, and N. Shen, Evaluation of the efficacy and safety of hydroxychloroquine in comparison with chloroquine in moderate and severe patients with COVID-19 Jan 2021, Science China Life Sciences, Volume 64, Issue 4, Page 660-663
LATE TREATMENT 28 patient HCQ late treatment study: 50% higher hospital discharge (p=0.09).
Small RCT comparing HCQ and CQ in China with 88 very late stage (17.6 days from onset to hospitalization and ~10 days to randomization) patients. The primary clinical outcomes (time to clinical recovery and time to clinical improvement) were not significantly different. Authors note that HCQ may have more promising efficacy in immune system modulation, indicated by ferritin reduction in the moderate cases and improvement of CT scores and lymphocyte counts in the severe cases. HCQ and CQ were well tolerated. Authors also compare RCT patients to a matched sample of non-RCT patients in the same hospital, showing shorter time to discharge with CQ/HCQ, but not statistically significant due to the small size. https://c19p.org/li3
170. J. Matangila, R. Nyembu, G. Telo, C. Ngoy, T. Sakobo, J. Massolo, B. Muyembe, R. Mvwala, C. Ilunga, E. Limbole, J. Ntalaja, and R. Kongo, Clinical characteristics of COVID-19 patients hospitalized at Clinique Ngaliema, a public hospital in Kinshasa, in the Democratic Republic of Congo: A retrospective cohort study Dec 2020, PLoS ONE, Volume 15, Issue 12, Page e0244272
LATE TREATMENT 160 patient HCQ late treatment study: 55% lower mortality (p=0.21).
55% lower death with HCQ+AZ. Retrospective 160 hospitalized patients in the Democratic Republic of Congo, 92% receiving HCQ+AZ, showing adjusted OR 0.24 [0.03-2.2]. https://c19p.org/matangila
171. S. Ozturk, K. Turgutalp, M. Arici, A. Odabas, M. Altiparmak, Z. Aydin, E. Cebeci, T. Basturk, Z. Soypacaci, G. Sahin, T. Elif Ozler, E. Kara, H. Dheir, N. Eren, G. Suleymanlar, M. Islam, M. Ogutmen, E. Sengul, Y. Ayar, M. Dolarslan, S. Bakirdogen, S. Safak, O. Gungor, I. Sahin, I. Mentese, O. Merhametsiz, E. Oguz, D. Genek, N. Alpay, N. Aktas, M. Duranay, S. Alagoz, H. Colak, Z. Adibelli, I. Pembegul, E. Hur, A. Azak, D. Taymez, E. Tatar, R. Kazancioglu, A. Oruc, E. Yuksel, E. Onan, K. Turkmen, N. Hasbal, A. Gurel, B. Yelken, T. Sahutoglu, M. Gok, N. Seyahi et al., Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey Dec 2020, Nephrology Dialysis Transplantation, Volume 35, Issue 12, Page 2083-2095
LATE TREATMENT 1,150 patient HCQ late treatment study: 44% lower mortality (p=0.14).
Retrospective 1210 hospitalized patients in Turkey focused on chronic kidney disease, hemodialysis and renal transplant patients, but also showing lower mortality with HCQ. Subject to confounding by indication. https://c19p.org/ozturk
172. G. Serrano, J. Rogado, C. Pangua, B. Obispo, A. Martin Marino, M. Perez-Perez, A. Lopez-Alfonso, and M. Lara, COVID-19 and lung cancer: What do we know? Sep 2020, Ann. Oncol., 2020, Sep, 31, S1026, Volume 31, Page S1026
LATE TREATMENT 22 patient HCQ late treatment study: 43% lower mortality (p=0.15).
Small retrospective study of 22 lung cancer patients, 14 treated with HCQ+AZ, showing HCQ+AZ mortality relative risk RR 0.57, p = 0.145. https://c19p.org/serrano
173. G. Bousquet, G. Falgarone, D. Deutsch, S. Derolez, M. Lopez-Sublet, F. Goudot, K. Amari, Y. Uzunhan, O. Bouchaud, and F. Pamoukdjian, ADL-dependency, D-Dimers, LDH and absence of anticoagulation are independently associated with one-month mortality in older inpatients with Covid-19 Jun 2020, Aging, 11306-11313, Volume 12, Issue 12, Page 11306-11313
LATE TREATMENT 108 patient HCQ late treatment study: 43% lower mortality (p=0.15).
Observational prospective 108 hospitalized patients 65 and older, showing HCQ mortality OR 0.49, p = 0.15. https://c19p.org/bousquet
174. F. Fontana, F. Giaroni, M. Frisina, G. Alfano, G. Mori, L. Lucchi, R. Magistroni, and G. Cappelli, SARS-CoV-2 infection in dialysis patients in northern Italy: a single-centre experience Jun 2020, Clinical Kidney J., 334–339
LATE TREATMENT 15 patient HCQ late treatment study: 50% lower mortality (p=0.53).
Very small observational study of 15 dialysis patients showing HCQ mortality RR 0.50, p = 0.53. https://c19p.org/fontana
175. F. Alberici, E. Delbarba, C. Manenti, L. Econimo, F. Valerio, A. Pola, C. Maffei, S. Possenti, B. Lucca, R. Cortinovis, V. Terlizzi, M. Zappa, C. Saccà, E. Pezzini, E. Calcaterra, P. Piarulli, A. Guerini, F. Boni, A. Gallico, A. Mucchetti, S. Affatato, S. Bove, M. Bracchi, E. Costantino, R. Zubani, C. Camerini, P. Gaggia, E. Movilli, N. Bossini, M. Gaggiotti, and F. Scolari, A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection May 2020, Kidney Int., 20-26, July 1, 2020, Volume 98, Issue 1, Page 20-26
LATE TREATMENT 94 patient HCQ late treatment study: 43% lower mortality (p=0.12).
Analysis of 94 hemodialysis COVID-19 positive patients, showing lower mortality with HCQ treatment, not reaching statistical significance. https://c19p.org/alberici
176. J. Frontera, J. Rahimian, S. Yaghi, M. Liu, A. Lewis, A. Havenon, S. Mainali, J. Huang, E. Scher, T. Wisniewski, A. Troxel, S. Meropol, L. Balcer, and S. Galetta, Treatment with Zinc is Associated with Reduced In-Hospital Mortality Among COVID-19 Patients: A Multi-Center Cohort Study Oct 2020, Research Square
LATE TREATMENT 3,473 patient HCQ late treatment PSM study: 37% lower mortality (p=0.02).
Retrospective 3,473 hospitalized patients showing lower mortality with HCQ+zinc. https://c19p.org/frontera
177. A. Omma, A. Erden, H. Apaydin, M. Aslan, H. Çamlı, E. Şahiner, S. Güven, B. Armağan, S. Karaahmetoğlu, I. Ates, and O. Kucuksahin, Hydroxychloroquine shortened hospital stay and reduced intensive care unit admissions in hospitalized COVID-19 patients Jan 2022, The J. Infection in Developing Countries, Volume 16, Issue 01, Page 25-31
LATE TREATMENT 393 patient HCQ late treatment study: 28% lower mortality (p=0.3), 50% lower ICU admission (p=0.004), and 17% shorter hospitalization (p=0.007).
Retrospective 393 hospitalized COVID-19 patients in Turkey, showing lower ICU admission and shorter hospitalization time with HCQ. There was no significant difference for mortality. Severity was higher in the HCQ group with greater baseline ventilation, high flow oxygen, fever, and dyspnea. https://c19p.org/omma
178. A. Patil, C. K, P. Shenoy, C. S, V. Haridas, S. Kumar, M. Daware, R. Janardana, B. Pinto, R. Subramaniam, N. S, Y. Singh, S. Singhai, R. Jois, V. Jain, S. C, B. Dharmanand, C. Dharmapalaiah, S. KN, V. Rao, and V. Shobha, A Prospective Longitudinal Study Evaluating The Influence of Immunosuppressives and Other Factors On COVID-19 in Autoimmune Rheumatic Diseases Aug 2021, Research Square
9,212 patient HCQ prophylaxis study: 66% lower mortality (p=0.1) and 9% fewer cases (p=0.43).
Prospective study of 9,212 autoimmune rheumatic disease patients showing lower mortality with HCQ, without reaching statistical significance. Authors incorrectly state “HCQ use did not influence occurrence of COVID-19 (RR = 0.909, CI (0.715,1.154), p = 0.432) or mortality (p = 0.097).” Case fatality rate for the autoimmune rheumatic disease patients was 4.6 times higher than in the general population from the same area. https://c19p.org/patil
179. R. Mehrizi, A. Golestani, M. Malekpour, H. Karami, M. Nasehi, M. Effatpanah, H. Ranjbaran, Z. Shahali, A. Sari, and R. Daroudi, Drug prescription patterns and their association with mortality and hospitalization duration in COVID-19 patients: insights from big data Dec 2023, Frontiers in Public Health, Volume 11
LATE TREATMENT 917,198 patient HCQ late treatment study: 26% lower mortality (p<0.0001).
Retrospective study of 917,198 hospitalized COVID-19 cases covered by the Iran Health Insurance Organization over 26 months showing that antithrombotics, corticosteroids, and antivirals reduced mortality while diuretics, antibiotics, and antidiabetics increased it. Confounding makes some results very unreliable. For example, diuretics like furosemide are often used to treat fluid overload, which is more likely in ICU or advanced disease requiring aggressive fluid resuscitation. Hospitalization length has increased risk of significant confounding, for example longer hospitalization increases the chance of receiving a medication, and death may result in shorter hospitalization. Mortality results may be more reliable. Confounding by indication is likely to be significant for many medications. Authors adjustments have very limited severity information (admission type refers to ward vs. ER department on initial arrival). We can estimate the impact of confounding from typical usage patterns, the prescription frequency, and attenuation or increase of risk for ICU vs. all patients. For HCQ, usage was likely focused early in the pandemic, but relatively uniform across severity of hospitalized Iranian patients. Confounding by time would be a major issue, with usage concentrated during the early period with higher mortality, however authors adjust by admission month, suggesting that residual confounding may not significantly change the result. C19early.com notes that the adjustment matches the expected confounding by time. https://c19p.org/mehrizi
180. J. Gómez, L. Pérez-Belmonte, M. Rubio-Rivas, J. Bascuñana, R. Quirós-López, M. Martínez, E. Hernandez, F. Roque-Rojas, M. Méndez-Bailón, and R. Gómez-Huelgas, Mortality risk factors in patients with SARS-CoV-2 infection and atrial fibrillation: Data from the SEMI-COVID-19 registry Oct 2022, Medicina Clínica
LATE TREATMENT 1,799 patient HCQ late treatment study: 36% lower mortality (p<0.0001).
Retrospective 1,799 hospitalized COVID-19 patients with atrial fibrillation in Spain, showing lower mortality with HCQ treatment in unadjusted results. https://c19p.org/gomez
181. R. Rubio-Sánchez, E. Lepe-Balsalobre, and M. Viloria-Peñas, Prognostic factors for the severity of SARS-CoV-2 infection Mar 2021, Advances in Laboratory Medicine / Avances en Medicina de Laboratorio, Volume 2, Issue 2, Page 253-258
LATE TREATMENT 197 patient HCQ late treatment study: 40% lower severe cases (p=0.02).
Retrospective 197 hospitalized COVID-19 patients in Spain, showing lower progression to pneumonia with HCQ in unadjusted results. https://c19p.org/rubiosanchez
182. N. Patel, X. Wang, X. Fu, Y. Kawano, C. Cook, K. Vanni, G. Qiann, E. Banasiak, E. Kowalski, Y. Zhang, J. Sparks, and Z. Wallace, Factors Associated with COVID-19 Breakthrough Infection in the Pre-Omicron Era Among Vaccinated Patients with Rheumatic Diseases: A Cohort Study Jul 2022, medRxiv
11,468 patient HCQ prophylaxis study: 46% fewer cases (p=0.001).
Retrospective 11,468 vaccinated rheumatic disease patients in the USA, showing lower risk of COVID-19 with HCQ/CQ use compared with all other treatments. Adjusted results are only provided with respect to specific other treatments. https://c19p.org/patel4
183. C. Hernandez-Cardenas, I. Thirion-Romero, N. Rivera-Martinez, P. Meza-Meneses, A. Remigio-Luna, and R. Perez-Padilla, Hydroxychloroquine for the treatment of severe respiratory infection by COVID-19: a randomized controlled trial Feb 2021, medRxiv
LATE TREATMENT 214 patient HCQ late treatment RCT: 12% lower mortality (p=0.66).
Very late stage RCT with 214 patients, mean SpO2 65%, 162 on mechanical ventilation, showing no significant difference in mortality. Patients not intubated at baseline show greater improvement, HR 0.43 [0.09-2.03]. Table 4 shows different results to the abstract – table 4 adjusted HR 0.80 [0.51-1.23], abstract HR 0.88 [0.51-1.53]. There was no significant difference in severe adverse events. https://c19p.org/hernandezcardenas
184. N. Bernaola, R. Mena, A. Bernaola, C. Carballo, A. Lara, C. Bielza, and P. Larrañaga, Observational Study of the Efficiency of Treatments in Patients Hospitalized with Covid-19 in Madrid Jul 2020, medRxiv
LATE TREATMENT 1,645 patient HCQ late treatment study: 17% lower mortality (p<0.0001).
HCQ HR 0.83 [0.77-0.89] based on propensity score matched retrospective analysis of 1,645 hospitalized patients. Prednisone HR 0.85 [0.82-0.88], 14 other medications showed either no significant benefit or a negative effect. https://c19p.org/bernaola
185. M. Salesi and M. Sedarat, Clinical signs, symptoms, and severity of COVID-19 in patients with rheumatic diseases during the COVID-19 epidemic Dec 2023, Immunopathologia Persa, Volume 10, Issue 1, Page e40568
77 patient HCQ prophylaxis study: 85% lower severe cases (p=0.003) and 18% fewer moderate/severe cases (p=0.35).
Retrospective study of 77 outpatients with rheumatic diseases diagnosed with COVID-19, showing lower risk of severe COVID-19 with HCQ use in unadjusted results. https://c19p.org/salesi
186. P. Liu, M. Zhang, J. Li, Y. Peng, S. Yu, and R. Wu, Factors affecting different COVID-19 outcomes in patients with systemic lupus erythematosus during the second pandemic wave of COVID-19 in China Feb 2024, Lupus
301 patient HCQ prophylaxis study: 39% lower severe cases (p=0.26).
Retrospective 301 consecutive SLE patients with COVID-19, showing lower risk of severe outcomes with HCQ use, with statistical significance in multivariable adjusted model 1 but not model 2. https://c19p.org/liu18
187. S. Huang, X. Ma, J. Cao, M. Du, Z. Zhao, D. Wang, X. Xu, J. Liang, and L. Sun, Effect of traditional therapeutics on prevalence and clinical outcomes of coronavirus disease 2019 in Chinese patients with autoimmune diseases Dec 2023, J. Translational Autoimmunity, Page 100227
432 patient HCQ prophylaxis study: 43% lower hospitalization (p=0.09) and 6% more cases (p=0.25).
Retrospective 432 autoimmune disease patients in China showing lower hospitalization with HCQ without statistical significance (OR 0.566, p=0.085) in unadjusted results, slightly higher COVID-19 cases without statistical significance, and increased cough compared with CNI. https://c19p.org/huang7
188. A. Rabe, W. Loke, R. Kalyani, R. Tummala, H. Stirnadel-Farrant, J. Were, and K. Winthrop, Impact of SARS-CoV-2 infection on patients with systemic lupus erythematosus in England prior to vaccination: a retrospective observational cohort study Nov 2023, BMJ Open, Volume 13, Issue 11, Page e071072
6,145 patient HCQ prophylaxis study: 29% fewer cases (p=0.22).
Retrospective cohort of 6,145 SLE patients showing lower incidence of COVID-19 for patients receiving HCQ/CQ (antimalarials), without statistical significance. Groups were not matched and results may be influenced by factors such as disease severity. HCQ/antimalarials were used more in moderate/severe SLE patients, suggesting that the estimated protective effect will underestimate the real effect. https://c19p.org/rabe
189. L. Dulcey, R. Caltagirone, J. Leon, F. Rangel, R. Strauch, V. Peña, M. Ciliberti, E. Blanco, Long-Term Hydroxychloroquine and Its Association with Covid-19 Infection, a Cohort Study from a South American Hospital May 2023, J. Clinical Rheumatology, Volume 29, Issue 4S1, Page S1-S112
967 patient HCQ prophylaxis study: 21% fewer cases (p=0.27).
PSM retrospective 322 rheumatological patients on HCQ and 645 matched controls, showing lower risk of COVID-19 with treatment, without statistical significance. Authors mention lower mortality with HCQ but do not provide details. Only an abstract is available. https://c19p.org/dulcey
190. C. Sukumar, N. Bolanthakodi, A. Venkatramanan, R. Nagraj, and S. Vidyasagar, The Frontline War: A Case-control study of risk factors for COVID-19 among health care workers Nov 2022, F1000Research, Volume 11, Page 1298
116 patient HCQ prophylaxis study: 38% fewer cases (p=0.3).
Case control study of healthcare workers in India, showing lower risk of cases with HCQ prophylaxis, without statistical significance. While authors comment negatively, as may be required for publication, and this study alone is not statistically significant, the result is consistent with the positive results in all studies to date. https://c19p.org/sukumar
191. K. Becetti, E. Satti, B. Varughese, Y. Al Rimawi, R. Sheikh Saleh, N. Hadwan, M. Gharib, M. Al Kahlout, E. Abuhelaiqa, H. Afif Ashour, R. Singh, and S. Emadi, Prevalence of coronavirus disease 2019 in a multiethnic cohort of patients with autoimmune rheumatic diseases in Qatar Aug 2022, Qatar Medical J., Volume 2022, Issue 3
700 patient HCQ prophylaxis study: 37% fewer cases (p=0.17).
Retrospective 700 patients with autoimmune rheumatic disease in Qatar, showing lower risk of COVID-19 with HCQ use, without statistical significance. For patients having close contact with COVID-19 cases, there was a statistically significant association with HCQ use and lower risk of COVID-19 in unadjusted results. https://c19p.org/becetti
192. E. Osawa and A. Maciel, Characteristics and risk factors for mortality in critically ill patients with COVID-19 receiving invasive mechanical ventilation: the experience of a private network in Sao Paulo, Brazil Jun 2022, The J. Critical Care Medicine, Volume 8, Issue 3, Page 165-175
LATE TREATMENT 215 patient HCQ ICU study: 29% lower mortality (p=0.07).
Retrospective 215 mechanically ventilated COVID-19 patients in Brazil, 71 treated with HCQ, showing lower mortality with treatment in unadjusted results, without statistical significance. Authors note HCQ was used more toward the start of the pandemic, which may introduce confounding due to overall protocols improving over time, suggesting that the actual benefit may be greater. https://c19p.org/osawa
193. L. Guglielmetti, D. Aschieri, I. Kontsevaya, F. Calabrese, A. Donisi, A. Faggi, P. Ferrante, E. Fronti, L. Gerna, M. Leoni, F. Paolillo, G. Ratti, A. Ruggieri, D. Sacchini, M. Scotti, C. Valdatta, M. Stabile, G. Taliani, and M. Codeluppi, Treatment for COVID-19—a cohort study from Northern Italy Oct 2021, Scientific Reports, Volume 11, Issue 1
LATE TREATMENT 600 patient HCQ late treatment study: 28% lower mortality (p=0.1).
Retrospective 600 hospitalized patients in Italy, showing lower mortality with HCQ treatment, without reaching statistical significance (p = 0.1). https://c19p.org/guglielmetti2
194. S. Bae, B. Ghang, Y. Kim, J. Lim, S. Yun, Y. Kim, S. Lee, and S. Kim, Recent Hydroxychloroquine Use Is Not Significantly Associated with Positive PCR Results for SARS-CoV-2: A Nationwide Observational Study in South Korea Feb 2021, Viruses 2021, Volume 13, Issue 2, Page 329
3,441 patient HCQ prophylaxis PSM study: 30% fewer cases (p=0.18).
Retrospective database analysis of prior HCQ usage in South Korea, showing non-statistically significantly lower mortality and cases with treatment. https://c19p.org/bae
196. S. Jung, M. Kim, M. Kim, S. Choi, J. Chung, and S. Choi, Effect of hydroxychloroquine pre-exposure on infection with SARS-CoV-2 in rheumatic disease patients: A population-based cohort study Dec 2020, Clinical Microbiology and Infection, Volume 27, Issue 4, Page 611-617
2,066 patient HCQ prophylaxis study: 59% lower mortality (p=1) and 13% more cases (p=0.86).
Retrospective cohort study of RA and SLE patients not showing a significant difference in PCR+ cases. PCR+ does not distinguish asymptomatic cases or severity. There was only one death which was in the control group. No other information on severity is provided. 33% of the control group used HCQ within the last year. Remaining confounding by differences in the nature and severity of rheumatic disease is likely. https://c19p.org/jung
197. L. Guglielmetti, I. Kontsevaya, M. Leoni, P. Ferrante, E. Fronti, L. Gerna, C. Valdatta, A. Donisi, A. Faggi, F. Paolillo, G. Ratti, A. Ruggieri, M. Scotti, D. Sacchini, G. Taliani, and M. Codeluppi, Severe COVID-19 pneumonia in Piacenza, Italy – a cohort study of the first pandemic wave Dec 2020, J. Infection and Public Health, Volume 14, Issue 2, Page 263-270
LATE TREATMENT 218 patient HCQ late treatment study: 35% lower mortality (p=0.22).
Retrospective 218 hospitalized patients in Italy showing non-statistically significant 35% lower mortality with HCQ, hazard ratio aHR 0.65 [0.33–1.30]. https://c19p.org/guglielmetti
198. B. Lambermont, M. Ernst, P. Demaret, S. Boccar, C. Gurdebeke, V. Cedric, M. Quinonez, C. Dubois, T. Lemineur, T. Njambou, B. Akando, D. Wertz, J. Higny, P. Delanaye, and B. Misset, Predictors of Mortality and Effect of Drug Therapies in Mechanically Ventilated Patients With Coronavirus Disease 2019: A Multicenter Cohort Study Nov 2020, Critical Care Explorations, Volume 2, Issue 12, Page e0305
LATE TREATMENT 247 patient HCQ late treatment study: 32% lower mortality (p=0.46).
Retrospective 247 mechanically ventilated patients showing lower mortality with HCQ, but not statistically significant on multiple Cox regression. The paper gives the p value for multiple Cox (0.46) and simple Cox (0.02), but does not specify the adjusted risk values. https://c19p.org/lambermont
199. C. Rodriguez-Gonzalez, E. Chamorro-de-Vega, M. Valerio, M. Amor-Garcia, F. Tejerina, M. Sancho-Gonzalez, A. Narrillos-Moraza, A. Gimenez-Manzorro, S. Manrique-Rodriguez, M. Machado, M. Olmedo, V. Escudero-Vilaplana, C. Villanueva-Bueno, B. Torroba-Sanz, A. Melgarejo-Ortuño, J. Vicente-Valor, A. Herranz, E. Bouza, P. Muñoz, and M. Sanjurjo, COVID-19 in hospitalized patients in Spain: a cohort study in Madrid Nov 2020, Int. J. Antimicrobial Agents, Volume 57, Issue 2, Page 106249
LATE TREATMENT 1,208 patient HCQ late treatment study: 23% lower mortality (p=0.26).
Retrospective 1255 patients in Spain showing lower mortality with HCQ. Subject to confounding by indication. https://c19p.org/rodriguezgonzalez
200. K. Van Halem, R. Bruyndonckx, J. Van der Hilst, J. Cox, P. Driesen, M. Opsomer, E. Van Steenkiste, B. Stessel, J. Dubois, and P. Messiaen, Risk factors for mortality in hospitalized patients with COVID-19 at the start of the pandemic in Belgium: a retrospective cohort study Nov 2020, BMC Infect Dis., Volume 20, Issue 1
LATE TREATMENT 319 patient HCQ late treatment study: 32% lower mortality (p=0.05).
Retrospective 319 hospitalized patients in Belgium showing lower mortality with HCQ. https://c19p.org/vanhalem
201. B. Revollo, C. Tebe, J. Peñafiel, I. Blanco, N. Perez-Alvarez, R. Lopez, L. Rodriguez, J. Ferrer, P. Ricart, E. Moret, C. Tural, A. Carreres, J. Matllo, S. Videla, B. Clotet, and J. Llibre, Hydroxychloroquine pre-exposure prophylaxis for COVID-19 in healthcare workers Nov 2020, J. Antimicrobial Chemotherapy, Volume 76, Issue 3, Page 827-829
487 patient HCQ prophylaxis PSM study: 23% fewer cases (p=0.52).
Retrospective PrEP analysis with 69 healthcare workers on PrEP HCQ, and 418 control. Authors report PCR and IgG results, with no baseline results for either. Authors note they “identified 69 HCWs receiving HCQ” while providing no information as to why or when they started HCQ. No conclusions can be drawn from this study because many workers may have been positive before starting HCQ. Only 14% of workers chose to use HCQ and they may have been motivated to do so because they had an infection. Authors perform several different adjustments, finding very different results. No information on death, hospitalization, symptoms, or severity is provided. Details on timing of serology and baseline serology status is not provided. Potential bias due to self-selection for risk. 25% of infections were detected before 7 days, indicating that they actually happened earlier (PCR false positive is very high initially). It is likely that many infections were before HCQ could reach therapeutic levels. https://c19p.org/revollo
202. D. Datta, S. Ghosal, B. Sinha, S. Datta, T. Chakraborty, K. Gangopadhyay, A. Dutta, No Role of HCQ in COVID-19 Prophylaxis: A Survey amongst Indian Doctors Nov 2020, J. Vaccines & Vaccination, S6:1000002
281 patient HCQ prophylaxis study: 22% fewer cases (p=0.47).
Survey of Indian doctors not finding a significant effect of HCQ prophylaxis. https://c19p.org/datta
203. P. Behera, B. Patro, A. Singh, P. Chandanshive, R. S. R., S. Pradhan, S. Pentapati, G. Batmanabane, P. Mohapatra, B. Padhy, S. Bal, S. Singh, and R. Mohanty, Role of ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: A matched case-control study Nov 2020, PLoS ONE, Volume 16, Issue 2, Page e0247163
372 patient HCQ prophylaxis study: 28% fewer cases (p=0.29).
Retrospective matched case-control prophylaxis study for HCQ, ivermectin, and vitamin C with 372 healthcare workers, showing lower COVID-19 incidence for all treatments, with statistical significance reached for ivermectin. HCQ OR 0.56, p = 0.29 ivermectin OR 0.27, p < 0.001 vitamin C OR 0.82, p = 0.58 https://c19p.org/behera
204. S. Ñamendys-Silva, P. Alvarado-Ávila, G. Domínguez-Cherit, E. Rivero-Sigarroa, L. Sánchez-Hurtado, A. Gutiérrez-Villaseñor, J. Romero-González, H. Rodríguez-Bautista, A. García-Briones, C. Garnica-Camacho, N. Cruz-Ruiz, M. González-Herrera, F. García-Guillén, M. Guerrero-Gutiérrez, J. Salmerón-González, L. Romero-Gutiérrez, J. Canto-Castro, and V. Cervantes, Outcomes of patients with COVID-19 in the Intensive Care Unit in Mexico: A multicenter observational study Oct 2020, Heart & Lung, Volume 50, Issue 1, Page 28-32
LATE TREATMENT 164 patient HCQ ICU study: 32% lower mortality (p=0.19).
Retrospective 164 ICU patients in Mexico showing 32% lower mortality with HCQ+AZ and 37% lower with CQ. HCQ+AZ vs. neither HCQ or CQ relative risk RR 0.68, p = 0.03 CQ vs. neither HCQ or CQ relative risk RR 0.63, p = 0.02 HCQ+AZ or CQ vs. neither relative risk RR 0.65, p = 0.006 https://c19p.org/namendyssilva
205. P. Guisado-Vasco, S. Valderas-Ortega, M. Carralón-González, A. Roda-Santacruz, L. González-Cortijo, G. Sotres-Fernández, E. Martí-Ballesteros, J. Luque-Pinilla, E. Almagro-Casado, F. La Coma-Lanuza, R. Barrena-Puertas, E. Malo-Benages, M. Monforte-Gómez, R. Diez-Munar, E. Merino-Lanza, L. Comeche-Casanova, M. Remirez-de-Esparza-Otero, M. Correyero-Plaza, M. Recio-Rodríguez, M. Rodríguez-López, M. Sánchez-Manzano, C. Andreu-Vázquez, I. Thuissard-Vasallo, J. María-Tomé, and D. Carnevali-Ruiz, Clinical characteristics and outcomes among hospitalized adults with severe COVID-19 admitted to a tertiary medical center and receiving antiviral, antimalarials, glucocorticoids, or immunomodulation with tocilizumab or cyclosporine: A retrospective observational study (COQUIMA cohort) Oct 2020, EClinicalMedicine, Volume 28, Page 100591
LATE TREATMENT 607 patient HCQ late treatment study: 20% lower mortality (p=0.36).
Retrospective 607 patients reporting results for early outpatient HCQ use with mortality odds ratio OR 0.092 [0.022-0.381], p = 0.001 (65 patients), and for hospital use, mortality odds ratio OR 0.737 [0.38-1.41], p = 0.36 (558 patients). Median age 69. https://c19p.org/guisadovasco
206. J. Piñana, R. Martino, I. García-García, R. Parody, M. Morales, G. Benzo, I. Gómez-Catalan, R. Coll, I. De La Fuente, A. Luna, B. Merchán, A. Chinea, D. De Miguel, A. Serrano, C. Pérez, C. Diaz, J. Lopez, A. Saez, R. Bailen, T. Zudaire, D. Martínez, M. Jurado, M. Calbacho, L. Vázquez, I. Garcia-Cadenas, L. Fox, A. Pimentel, G. Bautista, A. Nieto, P. Fernandez, J. Vallejo, C. Solano, M. Valero, I. Espigado, R. Saldaña, L. Sisinni, J. Ribera, M. Jimenez, M. Trabazo, M. Gonzalez-Vicent, N. Fernández, C. Talarn, M. Montoya, A. Cedillo, and A. Sureda, Risk factors and outcome of COVID-19 in patients with hematological malignancies Aug 2020, Experimental Hematology & Oncology, Volume 9, Issue 1
HCQ prophylaxis study: 36% lower mortality (p=0.11).
Retrospective study of 367 hematology patients with COVID-19 in Spain. Among 216 patients with very severe COVID-19, there was significantly lower mortality with azithromycin treatment. Mortality was also lower with HCQ, but without statistical significance. https://c19p.org/pinana
207. A. D’Arminio Monforte, A. Tavelli, F. Bai, G. Marchetti, and A. Cozzi-Lepri, Effectiveness of Hydroxychloroquine in COVID-19 disease: A done and dusted situation? Jul 2020, Int. J. Infectious Diseases, Volume 99, Page 75-76
LATE TREATMENT 539 patient HCQ late treatment study: 34% lower mortality (p=0.12).
HCQ+AZ adjusted death HR 0.44, p=0.009. Propensity scores include baseline COVID-19 disease severity, age, gender, number of comorbidities, cardio-vascular disease, duration of symptoms, date of admission, baseline plasma CRP. Inverse propensity weighting censoring. Retrospective study of 539 COVID-19 hospitalized patients in Milan, with treatment a median of 1 day after admission. HCQ 197 patients, HCQ+AZ 94, control 92. Control group received various other treatments. Authors excluded people receiving other drugs which could have biased the effect of HCQ when used in combination. Residual confounding is possible (e.g., people with CVD were more frequent in control), however people in the control group were more likely to require mechanical ventilation. https://c19p.org/darminiomonforte
208. P. Luo, L. Qiu, Y. Liu, X. Liu, J. Zheng, H. Xue, W. Liu, D. Liu, and J. Li, Metformin Treatment Was Associated with Decreased Mortality in COVID-19 Patients with Diabetes in a Retrospective Analysis May 2020, The American J. Tropical Medicine and Hygiene, Volume 103, Issue 1, Page 69-72
LATE TREATMENT 283 patient HCQ late treatment study: 32% lower mortality (p=0.72).
Retrospective 283 COVID-19+ diabetes patients in China, showing non-statistically significant lower mortality with HCQ/CQ treatment. https://c19p.org/luo3h
209. N. Capsoni, D. Privitera, A. Mazzone, C. Airoldi, V. Albertini, L. Angaroni, M. Bergamaschi, A. Molin, E. Forni, F. Pierotti, E. Rocca, F. Vincenti, and A. Bellone, CPAP Treatment In COVID-19 Patients: A Retrospective Observational Study In The Emergency Department Nov 2020, Research Square
LATE TREATMENT 52 patient HCQ late treatment study: 40% lower ventilation (p=0.3).
Small 52 patient retrospective study of patients with acute respiratory failure showing lower rates of intubation with HCQ. https://c19p.org/capsoni
210. T. Arleo, D. Tong, J. Shabto, G. O’Keefe, and A. Khosroshahi, Clinical Course and Outcomes of coronavirus disease 2019 (COVID-19) in Rheumatic Disease Patients on Immunosuppression: A case Cohort Study at a Single Center with a Significantly Diverse Population Oct 2020, medRxiv
70 patient HCQ prophylaxis study: 50% lower mortality (p=0.67).
Retrospective hospitalized rheumatic disease patients showing 50% lower mortality for patients on HCQ. https://c19p.org/arleo
211. L. Smith, N. Mendoza, D. Dobesh, and S. Smith, Observational Study on 255 Mechanically Ventilated Covid Patients at the Beginning of the USA Pandemic May 2021, medRxiv
LATE TREATMENT 255 patient HCQ late treatment study: 27% lower mortality (p=0.002).
Retrospective 255 mechanical ventilation patients in USA, showing that weight-adjusted HCQ+AZ improved survival by over 100%. QTc prolongation did not correlate with cumulative HCQ dose or HCQ serum level. Although authors mention immortal time bias, full details on the timing of HCQ administration is not provided and this is not fully addressed. Survival curves indicate immortal time bias will significantly change results, although the observed benefit appears to exceed the potential bias. https://c19p.org/smith
212. M. Ashraf, N. Shokouhi, E. Shirali, F. Davari-tanha, O. Memar, A. Kamalipour, A. Azarnoush, A. Mabadi, A. Ossareh, M. Sanginabadi, T. Azad, L. Aghaghazvini, S. Ghaderkhani, T. Poordast, A. Pourdast, and P. Nazemi, COVID-19 in Iran, a comprehensive investigation from exposure to treatment outcomes Apr 2020, medRxiv doi:10.1101/2020.04.20.20072421
EARLY TREATMENT 100 patient HCQ early treatment study: 68% lower mortality (p=0.15).
Small limited trial with 100 patients concluding that HCQ improved clinical outcome, OR 0.016 [0.002-0.11] in regression analysis. https://c19p.org/ashraf
213. M. Lecronier, A. Beurton, S. Burrel, L. Haudebourg, R. Deleris, J. Le Marec, S. Virolle, S. Nemlaghi, C. Bureau, P. Mora, M. De Sarcus, O. Clovet, B. Duceau, P. Grisot, M. Pari, J. Arzoine, U. Clarac, D. Boutolleau, M. Raux, J. Delemazure, M. Faure, M. Decavele, E. Morawiec, J. Mayaux, A. Demoule, and M. Dres, Comparison of hydroxychloroquine, lopinavir/ritonavir, and standard of care in critically ill patients with SARS-CoV-2 pneumonia: an opportunistic retrospective analysis Jul 2020, Critical Care, 2020, Volume 24, Issue 1
LATE TREATMENT 80 patient HCQ ICU study: 42% lower mortality (p=0.24), 6% lower treatment escalation (p=0.73), and 15% improved viral clearance (p=0.61).
Retrospective 80 ICU patients, 22 standard-of-care, 20 lopinavir/ritonavir, 38 HCQ. 28 day mortality 24% (HCQ) versus 41% (standard-of-care), a 41% decrease, but not statistically significant due to very small sample sizes. No statistically significant differences found for treatment escalation, ventilator-free days, viral load, or mortality. Authors consider treatment escalation more important than mortality, for unknown reasons. https://c19p.org/lecronier
214. H. Assad, Pharmacotherapy prescribing pattern and outcome for hospitalized patients with severe and critical COVID-19 Oct 2022, Current Issues in Pharmacy and Medical Sciences, Volume 0, Issue 0
LATE TREATMENT 291 patient HCQ late treatment study: 60% lower mortality (p=0.002).
Retrospective 346 hospitalized patients in Iraq, showing lower mortality with HCQ in unadjusted results. HCQ results are only provided within the 93% of patients treated with enoxaparin. https://c19p.org/assad
215. S. Samajdar, S. Mukherjee, T. Mandal, J. Paul, Ivermectin and Hydroxychloroquine for Chemo-Prophylaxis of COVID-19: A Questionnaire Survey of Perception and Prescribing Practice of Physicians vis-a-vis Outcomes Nov 2021, J. the Association of Physicians India
309 patient HCQ prophylaxis study: 75% fewer cases (p<0.0001).
Physician survey in India with 164 ivermectin prophylaxis, 129 HCQ prophylaxis, and 81 control patients, showing significantly lower COVID-19 cases with treatment. Details of the treatment and control groups and the definition of cases are not provided, and the results are subject to survey bias. Authors also report on community prophylaxis but present only combined ivermectin/HCQ results. https://c19p.org/samajdarh
216. I. Núñez-Gil, C. Fernández-Pérez, V. Estrada, V. Becerra-Muñoz, I. El-Battrawy, A. Uribarri, I. Fernández-Rozas, G. Feltes, M. Viana-Llamas, D. Trabattoni, J. López-País, M. Pepe, R. Romero, A. Castro-Mejía, E. Cerrato, T. Astrua, F. D’Ascenzo, O. Fabregat-Andres, J. Moreu, F. Guerra, J. Signes-Costa, F. Marín, D. Buosenso, A. Bardají, S. Raposeiras-Roubín, J. Elola, Á. Molino, J. Gómez-Doblas, M. Abumayyaleh, Á. Aparisi, M. Molina, A. Guerri, R. Arroyo-Espliguero, E. Assanelli, M. Mapelli, J. García-Acuña, G. Brindicci, E. Manzone, M. Ortega-Armas, M. Bianco, C. Trung, M. Núñez, C. Castellanos-Lluch, E. García-Vázquez, N. Cabello-Clotet, K. Jamhour-Chelh, M. Tellez, A. Fernández-Ortiz, and C. Macaya, Mortality risk assessment in Spain and Italy, insights of the HOPE COVID-19 registry Nov 2020, Intern. Emerg. Med., Volume 16, Issue 4, Page 957-966
LATE TREATMENT 954 patient HCQ late treatment study: 8% lower mortality (p=0.005).
Retrospective database study of 1,021 patients in Ecuador, Germany, Italy, and Spain, showing HCQ propensity score adjusted mortality odds ratio adjusted odds ratio 0.88, p=0.005. https://c19p.org/nunezgil
217. M. Maldonado, M. Ossorio, G. Del Peso, C. Santos, L. Álvarez, R. Sánchez-Villanueva, B. Rivas, C. Vega, R. Selgas, and M. Bajo, COVID-19 incidence and outcomes in a home dialysis unit in Madrid (Spain) at the height of the pandemic Nov 2020, Nefrología, Volume 41, Issue 3, Page 329-336
LATE TREATMENT 12 patient HCQ late treatment study: 91% lower mortality (p=0.17).
Very small retrospective of 12 dialysis patients showing 1/11 deaths with HCQ and 1/1 without HCQ. https://c19p.org/maldonado
218. R. Niwas, A. S, M. Garg, V. Nag, P. Bhatia, N. Dutt, N. Chauhan, J. Charan, S. Asfahan, P. Sharma, P. Bhardwaj, M. Banerjee, P. Garg, B. Sureka, G. Bohra, M. Gopalakrishnan, and S. Misra, Clinical outcome, viral response and safety profile of chloroquine in COVID-19 patients — initial experience Oct 2020, Advances in Respiratory Medicine, Volume 88, Issue 6, Page 515-519
LATE TREATMENT 29 patient HCQ late treatment study: 29% faster recovery (p=0.008).
Retrospective 12 hospitalized patients in India treated with CQ and 17 controls, showing faster recovery with treatment. There was no significant difference in viral clearance. The CQ group mean age was 41.3 vs. 47.6 for controls. https://c19p.org/niwas
219. B. Abella, E. Jolkovsky, B. Biney, J. Uspal, M. Hyman, I. Frank, S. Hensley, S. Gill, D. Vogl, I. Maillard, D. Babushok, A. Huang, S. Nasta, J. Walsh, E. Wiletyo, P. Gimotty, M. Milone, and R. Amaravadi, Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers Sep 2020, JAMA Internal Medicine, Volume 181, Issue 2, Page 195
125 patient HCQ prophylaxis RCT: 5% fewer cases (p=1).
Very small early-terminated underpowered PrEP RCT with 64/61 HCQ/control patients and only 8 infections, HCQ infection rate 6.3% versus control 6.6%, RR 0.95 [0.25 – 3.64]. There was no hospitalization or death, no significant difference in QTc, no severe adverse events, no cardiac events (e.g., syncope and arrhythmias) observed. Medication adherence was 81%. Therapeutic levels of HCQ may not have been reached by the time of the infection in the first week. 2 infections were reported to be after discontinuation of the medication, but the authors do not specify which arm these were in. Hypothetically, if these were both in the HCQ arm, the resulting RR for treatment would be much lower. https://c19p.org/abella
220. N. Alamdari, S. Afaghi, F. Rahimi, F. Tarki, S. Tavana, A. Zali, M. Fathi, S. Besharat, L. Bagheri, F. Pourmotahari, S. Irvani, A. Dabbagh, and S. Mousavi, Mortality Risk Factors among Hospitalized COVID-19 Patients in a Major Referral Center in Iran Sep 2020, Tohoku J. Exp. Med., 2020, 252, 73-84, Volume 252, Issue 1, Page 73-84
LATE TREATMENT 459 patient HCQ late treatment study: 55% lower mortality (p=0.03).
Retrospective 459 patients in Iran with 93% treated with HCQ, showing HCQ mortality RR 0.45, p = 0.028. HCQ was the only antiviral that showed a significant difference. There was relatively few control patients and the result is subject to confounding by indication. Average admission delay 5.72 days. https://c19p.org/alamdari
221. C. Santos, C. Morales, E. Álvarez, C. Castro, A. Robles, and T. Sandoval, Determinants of COVID-19 disease severity in patients with underlying rheumatic disease Jul 2020, Clinical Rheumatology, Volume 39, Issue 9, Page 2789-2796
38 patient HCQ prophylaxis study: 92% lower mortality (p=0.19).
Prospective study of 38 hospitalized rheumatic disease patients with COVID-19 in Spain, showing no mortality with existing HCQ use compared to 32% without, not reaching statistical significance. Authors also report on the use of HCQ/CQ after hospitalization. The prophylaxis and late treatment results are listed separately. https://c19p.org/santos
222. A. Cavalcanti, F. Zampieri, R. Rosa, L. Azevedo, V. Veiga, A. Avezum, L. Damiani, A. Marcadenti, L. Kawano-Dourado, T. Lisboa, D. Junqueira, P. De Barros e Silva, L. Tramujas, E. Abreu-Silva, L. Laranjeira, A. Soares, L. Echenique, A. Pereira, F. Freitas, O. Gebara, V. Dantas, R. Furtado, E. Milan, N. Golin, F. Cardoso, I. Maia, C. Hoffmann Filho, A. Kormann, R. Amazonas, M. Bocchi de Oliveira, A. Serpa-Neto, M. Falavigna, R. Lopes, F. Machado, and O. Berwanger, Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19 Jul 2020, NEJM, Volume 383, Issue 21, Page 2041-2052
LATE TREATMENT 667 patient HCQ late treatment RCT: 16% lower mortality (p=0.77) and 28% higher hospitalization (p=0.3).
Late stage RCT of 667 hospitalized patients with up to 14 days of symptoms at enrollment and receiving up to 4 liters per minute supplemental oxygen, not finding a significant effect after 15 days. Authors note: “the trial cannot definitively rule out either a substantial benefit of the trial drugs or a substantial harm”, sample sizes are too small. The paper uses the terms mild and moderate, however all patients had serious enough disease to be hospitalized, and 14% were actually randomized in the ICU. The trial had significant protocol deviations and unusually low medication adherence. Randomization resulted in 64.3% male patients (HCQ) vs. 54.2% (control) which may significantly affect results due to the much higher risk for male patients. Authors note: “our aim was to exclude patients already receiving longer and potentially therapeutic doses of the study treatments” in explanation for why the study protocol was changed to exclude patients with previous use of the medications >24hrs. Analyzing these patients rather than excluding them may have revealed effectiveness with early use as shown in other studies. The trial initially required enrollment within 48 hours of admission and was changed to remove this requirement, this change is likely to reduce effectiveness because enrollment was moved later, compared to the time the disease became serious enough for hospitalization. Total HCQ dosage 5.6g. A correction for 17 NEJM manuscript errors has been published including the statements: “The report as published did not provide accurate and complete information on the frequency and duration of previous use of hydroxychloroquine or azithromycin among the trial participants.” In the Participants subsection of Methods (page 2), the first sentence should have begun, “We enrolled patients who were either actively screened by the trial team or referred to us who were 18 years of age or older and had been hospitalized …,” rather than “The trial included consecutive patients who were 18 years of age or older and who had been hospitalized ….” In the second sentence, the phrase “previous use of chloroquine, hydroxychloroquine, azithromycin, or any other macrolide for more than 24 hours before enrollment (and since the onset of symptoms)” should have been omitted. In the third sentence, the phrase “, including criteria regarding previous use of hydroxychloroquine or azithromycin,” should have followed the term “exclusion criteria.” In the second paragraph of the Randomization, Interventions, and Follow-up subsection of Methods (page 2), the sentence beginning “The administration of hydroxychloroquine or chloroquine” should have been omitted. In the final footnote below Table 1 (page 4), the phrase “during the 24-hour period” should have been omitted, and the footnote should have ended, “Details are provided in the Supplementary Appendix.” In the Statistical Analysis subsection of Methods, in the final sentence of the paragraph beginning “We also performed …” (page 5), the phrase “during the randomized treatment period” should have been added after “medications received.” In the first paragraph of the Characteristics of the Patients subsection of Results (page 8), “randomization on May 17, 2020” should have been “ … on May 18, 2020.” At the end of that subsection, “Tables S5 and S6, respectively” should have been “Tables S5 through S7.” In the Primary Outcome subsection of Results (page 8), the mentions of Tables S7, S8, and S9, should have been Tables S8, S9, and S10, respectively. The final sentence of that subsection should have ended with “(Table S11) or in three post hoc subgroups defined according to the date of trial enrollment or according to previous use of hydroxychloroquine or azithromycin (Table S12),” rather than “(Table S10).” In the second paragraph of the Secondary Outcomes subsection of Results (page 8), the mentions of Table S11 and Table S12 should have been Table S13 and Table S14. In the final sentence of the Safety subsection of Results (page 9), the mention of Tables S13 and S14 should have been Tables S15 and S16. In the first sentence of the first footnote below Table 3 (page 11), the expression “during the randomized treatment period” should have been added after “according to the medications received.” In the penultimate paragraph of the Discussion (page 11), the sentence beginning, “The enrollment of patients with no previous use of these medications was challenging …” should have been replaced by, “We did not specify in our protocol the exclusion of such patients until late in the course of the trial, and as a consequence, 9.3% of the trial participants had previous use of hydroxychloroquine and 36.1% had previous use of azithromycin. However, in most instances, the duration of previous use was only 24 to 48 hours before enrollment, primarily because, before May 13, we required that patients be enrolled in the trial within 48 hours after hospital admission and because outpatient use of these drugs (before admission) was infrequent. After May 13, we specified that use of these drugs for more than 24 hours was an exclusion criterion.” The Supplementary Appendix was also affected. The extensive errata are illustrative of the poor quality control of the manuscript authorship, lack of proofreading (by the 34 different authors) and further suggests that little to no competent peer reviewing took place by the NEJM. https://c19p.org/cavalcanti
223. D. Boulware, M. Pullen, A. Bangdiwala, K. Pastick, S. Lofgren, E. Okafor, C. Skipper, A. Nascene, M. Nicol, M. Abassi, N. Engen, M. Cheng, D. LaBar, S. Lother, L. MacKenzie, G. Drobot, N. Marten, R. Zarychanski, L. Kelly, I. Schwartz, E. McDonald, R. Rajasingham, T. Lee, and K. Hullsiek, A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19 Jun 2020, NEJM, June 3 2020, Volume 383, Issue 6, Page 517-525
821 patient HCQ prophylaxis RCT: 17% fewer cases (p=0.35).
Remote post-exposure prophylaxis RCT reporting that “[HCQ] did not prevent illness compatible with Covid-19 or confirmed infection when used as postexposure prophylaxis within 4 days after exposure.” However, this statement is incorrect – cases were reduced, just without statistical significance – it’s not possible to conclude there was no efficacy. Additionally, treatment was not within 4 days – there was up to 68 hours shipping delay as below. Further, 6 independent analyses of the data in this study indicate efficacy. COVID-19 cases were reduced by [49%, 29%, 16%] respectively when taken within ~[70, 94, 118] hours of exposure (including shipping delay). The treatment delay-response relationship is significant at p=0.002. For more detailed analysis, see this NEJM article and analysis. Authors compare with treatment with folic acid, but folic acid is thought to bind to multiple SARS-CoV-2 proteins, folic acid levels are lower in COVID-19 patients with severe disease, folic acid supplementation may help with COVID-19 associated hypertension and hyperhomocystinemia, and differences in a folic acid-related enzyme could impact COVID-19 geographical severity variation. Time of dosing was not recorded in these trials. See Weisman et al, and Pullen et al data which shows shipping delay for these trials of 19 – 68 hours. With enrollment up to 4 days from exposure, this implies delivery 19 – 164 hours after exposure. https://c19p.org/boulwarepep
224. W. Hong, Y. Park, B. Kim, S. Park, J. Shin, S. Jang, H. Park, W. Yang, J. Jang, S. Jang, and T. Hwang, Use of combined treatment of 3rd-generation cephalosporin, azithromycin and antiviral agents on moderate SARs-CoV-2 patients in South Korea: A retrospective cohort study May 2022, PLOS ONE, Volume 17, Issue 5, Page e0267645
LATE TREATMENT 30 patient HCQ late treatment PSM study: 25% faster recovery (p=0.45), 13% longer hospitalization (p=0.75), and no change in viral clearance (p=0.99).
Retrospective 25 hospitalized patients treated with cephalosporin, azithromycin, and HCQ, and 217 standard-of-care patients in South Korea, reporting no significant differences. 5 patients receiving lopinavir/ritonavir and HCQ >5 days were excluded for unknown reasons. HCQ was typically initiated based on progression or side effects from another treatment. Conflicting results are reported. Table 2 indicates 15 CA/HCQ patients after matching, while Table S2 shows 25, and the Table 3 count is blank. S2 appears to incorrectly show before matching results, and the after matching results are missing in Table 3. 200mg HCQ bid is unadjusted. https://c19p.org/hong2
225. A. Bassets-Bosch, J. Raya-Muñoz, N. Wörner-Tomasa, S. Melendo-Pérez, and S. González-Peris, Negativización de PCR a SARS-CoV-2 en muestra respiratoria en pacientes con necesidad de asistencia recurrente Apr 2022, Anales de Pediatría, Volume 96, Issue 4, Page 357-359
LATE TREATMENT 15 patient HCQ late treatment study: 29% faster viral clearance (p=0.45).
Retrospective 15 pediatric patients in Spain, showing faster viral clearance with HCQ+AZ, without statistical significance. Treatment time and details are not provided. https://c19p.org/bassetsbosch
226. L. Rangel, P. Shah, K. Lo Sicco, A. Caplan, and A. Femia, Chronic Hydroxychloroquine Therapy and COVID-19 Outcomes: A Retrospective Case-Control Analysis Jan 2021, J. the American Academy of Dermatology, Volume 84, Issue 6, Page 1769-1772
153 patient HCQ prophylaxis study: 25% lower mortality (p=0.77) and 22% lower hospitalization (p=0.29).
Retrospective 50 COVID-19 patients that take chronic HCQ, compared to a matched sample of patients not taking chronic HCQ, showing lower mortality and ICU admission, and shorter hospitalization for HCQ patients, but not statistically significant due to the small number of events. The actual benefit for HCQ could be much larger. The study does not address the risk of being sick enough to visit the hospital. HCQ users are likely systemic autoimmune disease patients and authors do not adjust for the very different baseline risk for these patients. Other research shows that the risk of COVID-19 for systemic autoimmune disease patients is much higher overall, Ferri et al. show OR 4.42, p<0.001. https://c19p.org/rangel
227. E. Synolaki, V. Papadopoulos, G. Divolis, E. Gavriilidis, G. Loli, A. Gavriil, C. Tsigalou, O. Tsahouridou, E. Sertaridou, P. Rafailidis, A. Pasternack, D. Boumpas, G. Germanidis, O. Ritvos, S. Metallidis, P. Skendros, and P. Sideras, The Activin/Follistatin-axis is severely deregulated in COVID-19 and independently associated with in-hospital mortality Sep 2020, medRxiv
LATE TREATMENT 312 patient HCQ late treatment study: 24% lower mortality (p=0.27).
Retrospective 117 patients, 58 HCQ showing lower mortality for HCQ patients. Version 1 of this paper stated: “HCQ, AZ, [and …] were found to be independently associated with survival when treatment commenced at FACTCLINYCoD scores <3.” https://c19p.org/synolaki
228. M. González, E. Gonzalo, I. Lopez, F. Fernández, J. Pérez, D. Monge, J. Núñez, R. Fenoll, C. Fernández, S. Castro, M. Bailon, I. Fraile, M. Madrazo, P. Fontan, J. Gamboa, A. García, A. Vieitez, E. Aizpuru, A. Arostegui, A. Erdozain, C. Cilleros, J. Amigo, F. Epelde, C. Bermejo, and J. Santos, The Prognostic Value of Eosinophil Recovery in COVID-19: A Multicentre, Retrospective Cohort Study on Patients Hospitalised in Spanish Hospitals Aug 2020, medRxiv
LATE TREATMENT 9,644 patient HCQ late treatment study: 27% lower mortality (p=0.06).
Retrospective study focused on eosinophil recovery with 9,644 hospitalized patients in Spain, showing lower mortality for HCQ (14.7% vs 29.2%, p<0.001), and AZ (15.3% vs. 18.4%, p<0.001). With a multivariate model including potential confounding factors, HCQ and AZ are associated with lower mortality, HCQ OR 0.662, p=0.057. https://c19p.org/gonzalez2
229. J. Trullàs, E. Ruiz, C. Weisweiler, G. Badosa, A. Serra, H. Briceño, S. Soler, and J. Bisbe, High in-hospital mortality due to COVID-19 in a community hospital in Spain: a prospective observational study Jul 2020, Research Square
LATE TREATMENT 100 patient HCQ late treatment study: 36% lower mortality (p=0.12).
Retrospective 100 hospitalized patients in Spain showing lower mortality with HCQ+AZ. https://c19p.org/trullas
230. N. Klebanov, V. Pahalyants, J. Said, W. Murphy, N. Theodosakis, J. Scarry, S. Duey, M. Klevens, E. Lilly, and Y. Semenov, Antimalarials are not Effective as Pre-Exposure Prophylaxis for COVID-19: A Retrospective Matched Control Study Jun 2023, J. Drugs in Dermatology, Volume 22, Issue 8, Page 840-843
62,069 patient HCQ prophylaxis study: 31% lower mortality (p=0.8) and 6% more cases (p=0.7).
Retrospective 3,074 patients with antimalarial prescriptions and 58,955 matched controls, showing no significant differences with antimalarial prophylaxis for PCR+ cases (99% HCQ). Authors provide only PCR+ and mortality outcomes, and do not provide intermediate clinical outcomes that may show a statistically significant benefit. Authors do not adjust for the very different baseline risk for systemic autoimmune disease patients. Other research shows that the risk of COVID-19 for systemic autoimmune disease patients is much higher overall, Ferri et al. show OR 4.42, p<0.001 (for symptomatic disease). https://c19p.org/klebanov
231. K. Cárdenas-Jaén, S. Sánchez-Luna, A. Vaillo-Rocamora, M. Castro-Zocchi, L. Guberna-Blanco, D. Useros-Brañas, J. Remes-Troche, A. Ramos-De la Medina, B. Priego-Parra, J. Velarde-Ruiz Velasco, P. Martínez-Ayala, Á. Urzúa, D. Guiñez-Francois, K. Pawlak, K. Kozłowska-Petriczko, I. Gorroño-Zamalloa, C. Urteaga-Casares, I. Ortiz-Polo, A. Del Val Antoñana, E. Lozada-Hernández, E. Obregón-Moreno, G. García-Rayado, M. Domper-Arnal, D. Casas-Deza, E. Esteban-Cabello, L. Díaz, A. Riquelme, H. Martínez-Lozano, F. Navarro-Romero, I. Olivas, G. Iborra-Muñoz, A. Calero-Amaro, I. Caravaca-García, F. Lacueva-Gómez, R. Pastor-Mateu, B. Lapeña-Muñoz, V. Sastre-Lozano, N. Pizarro-Vega, L. Melcarne, M. Pedrosa-Aragón, J. Mira, A. MStat, I. Carrillo, and E. De-Madaria, Gastrointestinal symptoms and complications in patients hospitalized due to COVID-19, an international multicentre prospective cohort study (TIVURON project) Jun 2023, Gastroenterología y Hepatología, Volume 46, Issue 6, Page 425-438
LATE TREATMENT 829 patient HCQ late treatment study: 56% lower severe cases (p=0.13).
Retrospective 829 hospitalized COVID-19 patients in Spain focused on gastrointestinal symptoms, showing lower risk of severe COVID-19 with HCQ treatment in bivariate analysis, without statistical significance. https://c19p.org/cardenasjaen
232. W. Hafez, H. Saleh, Z. Al Baha, M. Tariq, S. Hamdan, and S. Ahmed, Antiviral Used among Non-Severe COVID-19 Cases in Relation to Time till Viral Clearance: A Retrospective Cohort Study Apr 2022, Antibiotics, Volume 11, Issue 4, Page 498
LATE TREATMENT 1,486 patient HCQ late treatment study: 12% faster viral clearance (p=0.59).
Retrospective hospitalized patients in the United Arab Emirates, showing no significant difference in viral clearance with different combinations of HCQ, AZ, favipiravir, and lopinavir/ritonavir. https://c19p.org/hafez
233. A. Beaumont, D. Vignes, R. Sterpu, G. Bussone, I. Kansau, C. Pignon, R. Ben Ismail, M. Favier, J. Molitor, D. Braham, R. Fior, S. Roy, M. Mion, L. Meyer, M. Andronikof, C. Damoisel, P. Chagué, J. Aurégan, N. Bourgeois-Nicolaos, C. Guillet-Caruba, J. Téglas, and S. Abgrall, Factors associated with hospital admission and adverse outcome for COVID-19: role of social factors and medical care Feb 2022, Infectious Diseases Now
LATE TREATMENT 296 patient HCQ late treatment study: 14% lower combined mortality/intubation (p=0.55).
Retrospective 296 hospitalized patients in France, showing no significant difference with HCQ treatment. https://c19p.org/beaumont
234. H. Uygun, Effect of Hydroxychloroquine Use on the Length Of Hospital Stay in Children Diagnosed With Covid 19 Sep 2021, Northern Clinics of Istanbul
LATE TREATMENT 40 patient HCQ late treatment study: 12% faster viral clearance (p=0.05).
Retrospective 40 pediatric hospitalized patients, 15 treated with HCQ, showing 7.2 vs. 8.2 days until PCR-, not quite reaching statistical significance. https://c19p.org/uygen
235. M. Gonenli, I. Kayi, N. Alpay-Kanitez, T. Baydas, M. Kose, E. Nalbantoglu, M. Keskinler, T. Akpinar, and O. Ergonul, Analysis of the Prophylactic use of Hydroxychloroquine at the Beginning of the COVID-19 Pandemic Among Physicians Dec 2020, Infectious Diseases and Clinical Microbiology, Volume 4, Issue 4, Page 236-243
564 patient HCQ prophylaxis study: 30% lower progression (p=0.77) and 19% more cases (p=0.58).
Small prophylaxis survey showing lower, but not statistically significant, progression to pneumonia (3 of 148 HCQ, 12 of 416 control), RR 0.70, p = 0.77. There was a higher incidence of cases with HCQ, OR 1.19, p = 0.58, which may be due to survey bias, treatment self-selection, and inconsistent regimens. Improvement on severity may be related to the higher HCQ concentration in lung tissue, and also reflect that binary PCR does not distinguish replication-competence. Details of the pneumonia numbers for treatment/control are from the author, it’s unclear why the lower progression to pneumonia was not reported in the paper. https://c19p.org/gonenli
236. L. Orioli, T. Servais, L. Belkhir, P. Laterre, J. Thissen, B. Vandeleene, D. Maiter, J. Yombi, and M. Hermans, Clinical characteristics and short-term prognosis of in-patients with diabetes and COVID-19: A retrospective study from an academic center in Belgium Dec 2020, Diabetes & Metabolic Syndrome: Clinical Research & Reviews, Volume 15, Issue 1, Page 149-157
LATE TREATMENT 73 patient HCQ late treatment study: 13% lower mortality (p=1).
Small retrospective study of 73 diabetic patients in Belgium, 55 HCQ patients, showing HCQ RR 0.87, p = 1.0. https://c19p.org/orioli
237. S. Peng, H. Wang, X. Sun, P. Li, Z. Ye, Q. Li, J. Wang, X. Shi, L. Liu, Y. Yao, R. Zeng, F. He, J. Li, S. Ge, X. Ke, Z. Zhou, E. Dong, H. Wang, G. Xu, L. Zhang, and M. Zhao, Early versus late acute kidney injury among patients with COVID-19—a multicenter study from Wuhan, China Dec 2020, Nephrology Dialysis Transplantation, Volume 35, Issue 12, Page 2095-2102
LATE TREATMENT 4,020 patient HCQ late treatment study: 11% lower progression (p=0.63).
Retrospective 4020 hospitalized patients in China showing non-statistically significant lower risk of acute kidney injury with HCQ. https://c19p.org/peng
238. A. Rodríguez, G. Moreno, J. Gómez, R. Carbonell, E. Picó-Plana, C. Benavent Bofill, R. Sánchez Parrilla, S. Trefler, E. Esteve Pitarch, L. Canadell, X. Teixido, L. Claverias, and M. Bodí, Severe infection due to the SARS-CoV-2 coronavirus: Experience of a tertiary hospital with COVID-19 patients during the 2020 pandemic Nov 2020, Medicina Intensiva, Volume 44, Issue 9, Page 525-533
LATE TREATMENT 43 patient HCQ late treatment study: 59% lower mortality (p=0.23).
Small prospective study of 43 hospitalized patients with 39 taking HCQ, showing unadjusted mortality relative risk RR 0.41, p=0.23. https://c19p.org/rodriguez
239. M. Rivera-Izquierdo, M. Valero-Ubierna, J. R-delAmo, M. Fernández-García, S. Martínez-Diz, A. Tahery-Mahmoud, M. Rodríguez-Camacho, A. Gámiz-Molina, N. Barba-Gyengo, P. Gámez-Baeza, C. Cabrero-Rodríguez, P. Guirado-Ruiz, D. Martín-Romero, A. Láinez-Ramos-Bossini, M. Sánchez-Pérez, J. Mancera-Romero, M. García-Martín, L. Martín-delosReyes, V. Martínez-Ruiz, and E. Jiménez-Mejías, Agentes terapéuticos utilizados en 238 pacientes hospitalizados por COVID-19 y su relación con la mortalidad Jul 2020, Medicina Clínica, Volume 155, Issue 9, Page 375-381
LATE TREATMENT 238 patient HCQ late treatment study: 19% lower mortality (p=0.75).
Retrospective 238 hospitalized patients in Spain showing lower mortality with HCQ, adjusted hazard ratio 0.81 [0.24-2.76]. https://c19p.org/riveraizquierdo
240. O. Paccoud, F. Tubach, A. Baptiste, A. Bleibtreu, D. Hajage, G. Monsel, G. Tebano, D. Boutolleau, E. Klement, N. Godefroy, R. Palich, O. Itani, A. Faiçal, M. Valantin, R. Tubiana, S. Burrel, V. Calvez, E. Caumes, A. Marcelin, and V. Pourcher, Compassionate use of hydroxychloroquine in clinical practice for patients with mild to severe Covid-19 in a French university hospital Jun 2020, Clinical Infectious Diseases, Volume 73, Issue 11, Page e4064-e4072
LATE TREATMENT 89 patient HCQ late treatment study: 11% lower mortality (p=0.88).
Retrospective of 89 hospitalized patients, survival HR 0.89 [0.23-3.47], not statistically significant. Authors note that unmeasured confounders may have persisted (no propensity score matching analysis calculated) and the study may be underpowered. https://c19p.org/paccoud
241. S. Hraiech, J. Bourenne, K. Kuteifan, J. Helms, J. Carvelli, M. Gainnier, F. Meziani, and L. Papazian, Lack of viral clearance by the combination of hydroxychloroquine and azithromycin or lopinavir and ritonavir in SARS-CoV-2-related acute respiratory distress syndrome May 2020, Ann. Intensive Care, Volume 10, Issue 1
LATE TREATMENT 32 patient HCQ ICU study: 65% lower mortality (p=0.21) and 3% worse viral clearance (p=1).
Retrospective 45 ICU patients, 17 treated with HCQ+AZ, showing no significant difference in viral clearance after 6 days, or mortality 6 days from acute respiratory distress syndrome. https://c19p.org/hraiech
242. J. Magagnoli, S. Narendran, F. Pereira, T. Cummings, J. Hardin, S. Sutton, and J. Ambati, Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 Apr 2020, Med, Volume 1, Issue 1, Page 114-127.e3
LATE TREATMENT 807 patient HCQ late treatment study: 11% lower mortality (p=0.74).
Retrospective 807 hospitalized patients, no statistically significant reduction in mortality or the need for mechanical ventilation with HCQ or HCQ+AZ, or for death with HCQ+AZ, HR 1.83, p=0.009 for HCQ mortality. The preprint notes that HCQ was more likely to be prescribed to patients with more severe disease, however this was deleted in the published version. 425 patients had dispositions of death or discharge by the end of the study period and thus did not encounter the issue of length-biased sampling and differential rates of right-censored observations among the groups. Also see this piece on accusations of “spectacular” scientific misconduct by article author University of South Carolina Pharmacy School Professor Joseph Magagnoli, and additionally illustrative of poor or incompetent journal peer review process. https://c19p.org/magagnoli
243. S. Yegorov, M. Goremykina, R. Ivanova, S. Good, D. Babenko, A. Shevtsov, K. MacDonald, and Y. Zhunussov, Epidemiological and Clinical Characteristics, and Virologic Features of COVID-19 Patients in Kazakhstan: a Nation-Wide, Retrospective, Cohort Study Jan 2021, medRxiv
LATE TREATMENT 1,072 patient HCQ late treatment study: 95% lower mortality (p=1).
Retrospective 1,072 hospitalized patients in Kazakhstan showing no mortality for HCQ treated patients, however only 23 patients received treatment – this result is not statistically significant. https://c19p.org/yegerov
244. P. Soto-Becerra, C. Culquichicón, Y. Hurtado-Roca, and R. Araujo-Castillo, Real-World Effectiveness of hydroxychloroquine, azithromycin, and ivermectin among hospitalized COVID-19 patients: Results of a target trial emulation using observational data from a nationwide Healthcare System in Peru Oct 2020, medRxiv
LATE TREATMENT 3,322 patient HCQ late treatment study: 18% lower mortality (p<0.0001).
Retrospective database study of 5683 patients, 692 received HCQ/CQ+AZ, 200 received HCQ/CQ, 203 received ivermectin, 1600 received AZ, 358 received ivermectin+AZ, and 2630 received standard of care. This study includes anyone with ICD-10 COVID-19 codes which includes asymptomatic PCR+ patients, therefore many patients in the control group are likely asymptomatic with regards to SARS-CoV-2, but in the hospital for another reason. For those that had symptomatic COVID-19, there is also likely significant confounding by indication. In this study all medications show higher mortality at day 30, which is consistent with asymptomatic (for COVID-19) or mild condition patients being more common in the control group. For ivermectin they show 30 day mortality aHR = 1.39 [0.88 – 2.22]. KM curves show that the treatment groups were in more serious condition, and also that after about day 35 survival became better with ivermectin. The last day available for ivermectin shows RR 0.83, p = 0.01. More than the total excess mortality happened on the first day. This is consistent with treated patients being in more serious condition, and with many of the control group patients being in hospital for something unrelated to COVID-19. Authors use a machine learning based propensity scoring system that appears over-parameterized and likely to result in significant overfitting and inaccurate results. Essentially, they test for all interactions between two and three covariates. The nature and large number of covariates means many random correlations may be found. COVID-19 severity is not used. This study also does not compare treatments with a control group not receiving the treatment – authors put patients receiving treatments after 48 hours in the control group. Authors state that outcomes within 24 hours were excluded, however KM curves show significant mortality at day 1 (only for the treatment groups). Several protocol violations and missing data have also been reported in this study. Substantial unadjusted confounding by indication likely; includes PCR+ patients that may be asymptomatic for COVID-19 but are inpatient for other reasons. https://c19p.org/sotobecerra
245. A. Shoaibi, S. Fortin, R. Weinstein, J. Berlin, and P. Ryan, Comparative Effectiveness of Famotidine in Hospitalized COVID-19 Patients Sep 2020, medRxiv
LATE TREATMENT 29,451 patient HCQ late treatment study: 15% lower mortality (p=0.001).
Retrospective database analysis focused on Famotidine but also showing results for HCQ users, with unadjusted mortality RR 0.85, p<0.001 (13.6% vs. 16.1%). https://c19p.org/shoaibi
246. K. Fung, S. Baik, F. Baye, Z. Zheng, V. Huser, and C. McDonald, Effect of common maintenance drugs on the risk and severity of COVID-19 in elderly patients Sep 2021, PLoS ONE, Volume 17, Issue 4, Page e0266922
HCQ prophylaxis study: 13% lower mortality (p=0.15), 3% lower hospitalization (p=0.63), and 9% fewer cases (p=0.02).
Retrospective database analysis of 374,229 patients in the USA, showing no significant difference with HCQ use, however authors do not adjust for the very different baseline risk for systemic autoimmune disease patients. Other research shows that the risk of COVID-19 for systemic autoimmune disease patients is much higher overall, Ferri et al. show OR 4.42, p<0.001. Authors compare with patients that never used HCQ and with patients that previously used HCQ. https://c19p.org/fung
247. D. De Gonzalo-Calvo, M. Molinero, I. Benítez, M. Perez-Pons, N. García-Mateo, A. Ortega, T. Postigo, M. García-Hidalgo, T. Belmonte, C. Rodríguez-Muñoz, J. González, G. Torres, C. Gort-Paniello, A. Moncusí-Moix, Á. Estella, L. Tamayo Lomas, A. Martínez de la Gándara, L. Socias, Y. Peñasco, M. De la Torre, E. Bustamante-Munguira, E. Gallego Curto, I. Martínez Varela, M. Martin Delgado, P. Vidal-Cortés, J. López Messa, F. Pérez-García, J. Caballero, J. Añón, A. Loza-Vázquez, N. Carbonell, J. Marin-Corral, R. Jorge García, C. Barberà, A. Ceccato, L. Fernández-Barat, R. Ferrer, D. Garcia-Gasulla, J. Lorente-Balanza, R. Menéndez, A. Motos, O. Peñuelas, J. Riera, J. Bermejo-Martin, A. Torres, and F. Barbé, A blood microRNA classifier for the prediction of ICU mortality in COVID-19 patients: a multicenter validation study Jun 2023, Respiratory Research, Volume 24, Issue 1
LATE TREATMENT 491 patient HCQ ICU study: 38% lower mortality (p=0.23).
Retrospective 491 ICU patients in Spain showing lower mortality with HCQ without statistical significance in unadjusted results. https://c19p.org/degonzalocalvo
248. A. Fernández-Cruz, A. Puyuelo, L. Núñez Martín-Buitrago, E. Sánchez-Chica, C. Díaz-Pedroche, R. Ayala, M. Lizasoain, R. Duarte, C. Lumbreras, and J. Antonio Vargas, Higher mortality of hospitalized haematologic patients with COVID-19 compared to non-haematologic is driven by thrombotic complications and development of ARDS: An age-matched cohorts study Jan 2022, Clinical Infection in Practice, Volume 13, Page 100137
LATE TREATMENT 71 patient HCQ late treatment study: 27% lower mortality (p=0.47).
Retrospective 71 hospitalized haematologic patients in Spain, showing lower mortality with HCQ treatment in unadjusted results and without statistical significance. https://c19p.org/fernandezcruz
249. G. Menardi, L. Infante, V. Del Bono, L. Fenoglio, D. Collotta, P. Macagno, C. Bedogni, M. Rebora, C. Fruttero, and M. Collino, A retrospective analysis on pharmacological approaches to COVID-19 patients in an Italian hub hospital during the early phase of the pandemic Sep 2021, PharmAdvances, Volume 3, Issue 3, Page 576
LATE TREATMENT 277 patient HCQ late treatment study: 35% lower mortality (p=0.12).
Retrospective 277 hospitalized patients in Italy, showing lower mortality with HCQ treatment, not reaching statistical significance, and subject to confounding by indication. https://c19p.org/menardi
250. M. Mahto, A. Banerjee, B. Biswas, S. Kumar, N. Agarwal, P. Singh, Seroprevalence of IgG against SARS-CoV-2 and its determinants among healthcare workers of a COVID-19 dedicated hospital of India Feb 2021, American J. Blood Research
689 patient HCQ prophylaxis study: 27% lower IgG positivity (p=0.38).
Retrospective 689 healthcare workers in India, showing no significant difference in IgG positivity with HCQ prophylaxis in unadjusted results. https://c19p.org/mahto
251. B. Purandare, P. Rajhans, S. Jog, P. Dalvi, P. Prayag, P. Marudwar, H. Pawar, B. Pawar, N. Mahale, V. Narasimhan, G. Oak, S. Marreddy, A. Bedekar, P. Akole, B. Bhurke, S. Chavan, V. Telbhare, D. Diwane, M. Shahane, A. Prayag, S. Gugale, and S. Bhor, A Retrospective Observational Study of Hypoxic COVID-19 Patients Treated with Immunomodulatory Drugs in a Tertiary Care Hospital Dec 2020, Indian J. Critical Care Medicine, Volume 24, Issue 11, Page 1020-1027
LATE TREATMENT 134 patient HCQ late treatment study: 29% lower mortality (p=0.36).
Retrospective 134 hospitalized COVID-19 patients in India, showing no significant difference with HCQ treatment in unadjusted results. https://c19p.org/mahaleh
252. A. Chari, M. Samur, J. Martinez-Lopez, G. Cook, N. Biran, K. Yong, V. Hungria, M. Engelhardt, F. Gay, A. García Feria, S. Oliva, R. Oostvogels, A. Gozzetti, C. Rosenbaum, S. Kumar, E. Stadtmauer, H. Einsele, M. Beksac, K. Weisel, K. Anderson, M. Mateos, P. Moreau, J. San-Miguel, N. Munshi, and H. Avet-Loiseau, Clinical features associated with COVID-19 outcome in multiple myeloma: first results from the International Myeloma Society data set Dec 2020, Blood, Volume 136, Issue 26, Page 3033-3040
LATE TREATMENT 502 patient HCQ late treatment study: 33% lower mortality (p=0.17).
Retrospective multiple myeloma patients showing lower mortality with HCQ treatment, unadjusted RR 0.67, p = 0.17 (data is in the supplementary material). https://c19p.org/chari
253. R. Bielza, J. Sanz, F. Zambrana, E. Arias, E. Malmierca, L. Portillo, I. Thuissard, A. Lung, M. Neira, M. Moral, C. Andreu-Vázquez, A. Esteban, M. Ramírez, L. González, G. Carretero, R. Moreno, P. Martínez, J. López, M. Esteban-Ortega, I. García, M. Vaquero, A. Linares, A. Gómez-Santana, and J. Gómez Cerezo, Clinical characteristics, frailty and mortality of residents with COVID-19 in nursing homes of a region of Madrid Dec 2020, J. the American Medical Directors Association, Volume 22, Issue 2, Page 245-252.e2
LATE TREATMENT 630 patient HCQ late treatment study: 22% lower mortality (p=0.09).
Retrospective 630 elderly patients in Spain showing lower mortality with HCQ treatment, unadjusted relative risk RR 0.78, p = 0.09. HCQ was used more often with patients that were hospitalized (24% versus 3% use in the nursing homes). Median age 87. https://c19p.org/bielza
254. W. Qin, F. Dong, Z. Zhang, B. Hu, S. Chen, Z. Zhu, F. Li, X. Wang, Y. Zhang, Y. Wang, K. Zhen, J. Wang, I. Elalamy, C. Li, Z. Zhai, B. Davidson, and C. Wang, Low molecular weight heparin and 28-day mortality among patients with coronavirus disease 2019: A cohort study in the early epidemic era Nov 2020, Thrombosis Research, Volume 198, Page 19-22
LATE TREATMENT 749 patient HCQ late treatment study: 34% lower mortality (p=0.61).
Low molecular weight heparin study also showing results for HCQ treatment, unadjusted HCQ mortality relative risk RR 0.66, p = 0.61. https://c19p.org/qin
255. C. Santos, C. Morales, E. Álvarez, C. Castro, A. Robles, and T. Sandoval, Determinants of COVID-19 disease severity in patients with underlying rheumatic disease Jul 2020, Clinical Rheumatology, Volume 39, Issue 9, Page 2789-2796
LATE TREATMENT 38 patient HCQ late treatment study: 26% lower mortality (p=0.6).
Prospective study of 38 hospitalized rheumatic disease patients with COVID-19 in Spain, showing no mortality with existing HCQ use compared to 32% without, not reaching statistical significance. Authors also report on the use of HCQ/CQ after hospitalization. The prophylaxis and late treatment results are listed separately. https://c19p.org/santos2
256. S. Krishnan, K. Patel, R. Desai, A. Sule, P. Paik, A. Miller, A. Barclay, A. Cassella, J. Lucaj, Y. Royster, J. Hakim, Z. Ahmed, and F. Ghoddoussi, Clinical comorbidities, characteristics, and outcomes of mechanically ventilated patients in the State of Michigan with SARS-CoV-2 pneumonia Jul 2020, J Clin Anesth., Volume 67, Page 110005
LATE TREATMENT 152 patient HCQ late treatment study: 20% lower mortality (p=0.48).
Retrospective 152 mechanically ventilated patients in the USA showing unadjusted lower mortality with vitamin C, vitamin D, HCQ, and zinc treatment, statistically significant only for vitamin C. https://c19p.org/krishnan
257. J. Martínez-López, M. Mateos, C. Encinas, A. Sureda, J. Hernández-Rivas, A. Lopez de la Guía, D. Conde, I. Krsnik, E. Prieto, R. Riaza Grau, M. Gironella, M. Blanchard, N. Caminos, C. Fernández de Larrea, M. Senin, F. Escalante, J. De la Puerta, E. Giménez, P. Martínez-Barranco, J. Mateos, L. Casado, J. Bladé, J. Lahuerta, J. De la Cruz, and J. San-Miguel, Multiple Myeloma and SARS-CoV-2 Infection: Clinical Characteristics and Prognostic Factors of Inpatient Mortality Jun 2020, Blood Cancer J., Volume 10, Issue 10
LATE TREATMENT 167 patient HCQ late treatment study: 33% lower mortality (p=0.2).
Retrospective 167 multiple myeloma patients in Spain, showing no significant difference in mortality with HCQ treatment in unadjusted results without group details. https://c19p.org/martinezlopez
258. J. Goldman, D. Lye, D. Hui, K. Marks, R. Bruno, R. Montejano, C. Spinner, M. Galli, M. Ahn, R. Nahass, Y. Chen, D. SenGupta, R. Hyland, A. Osinusi, H. Cao, C. Blair, X. Wei, A. Gaggar, D. Brainard, W. Towner, J. Muñoz, K. Mullane, F. Marty, K. Tashima, G. Diaz, and A. Subramanian, Remdesivir for 5 or 10 Days in Patients with Severe Covid-19 May 2020, NEJM, Volume 383, Issue 19, Page 1827-1837
LATE TREATMENT 397 patient HCQ late treatment study: 22% lower mortality (p=0.46).
Study focused on remdesivir but with results for HCQ in the supplementary appendix, showing 9% death with HCQ versus 12% control, unadjusted relative risk uRR 0.78, p = 0.46. https://c19p.org/goldmanh
259. M. Martin-Vicente, R. Almansa, I. Martínez, A. Tedim, E. Bustamante, L. Tamayo, C. Aldecoa, J. Gómez, G. Renedo, J. Berezo, J. Cedeño, N. Mamolar, P. Olivares, R. Herrán, R. Cicuendez, P. Enríquez, A. Ortega, N. Jorge, A. De la Fuente, J. Bustamante-Munguira, M. Muñoz-Gómez, M. González-Rivera, C. Puertas, V. Más, M. Vázquez, F. Pérez-García, J. Rico-Feijoo, S. Martín, A. Motos, L. Fernandez-Barat, J. Eiros, M. Dominguez-Gil, R. Ferrer, F. Barbé, D. Kelvin, J. Bermejo-Martin, S. Resino, and A. Torres, Absent or insufficient anti-SARS-CoV-2 S antibodies at ICU admission are associated to higher viral loads in plasma, antigenemia and mortality in COVID-19 patients Mar 2021, medRxiv
LATE TREATMENT 92 patient HCQ ICU study: 59% lower mortality (p=0.41).
Retrospective 92 ICU patients with almost all treated with HCQ and only one non-HCQ treated patient that died, showing unadjusted non-statistically significant lower mortality with treatment. https://c19p.org/martinvicente
260. R. Alqassieh, I. Bsisu, M. Al-Sabbagh, N. El-Hammuri, M. Yousef, M. El Jarbeh, A. Sharqawi, H. Smadi, S. Abu-Halaweh, and M. Abufaraj, Clinical characteristics and predictors of the duration of hospital stay in COVID-19 patients in Jordan Dec 2020, F1000Research, Volume 9, Page 1439
LATE TREATMENT 131 patient HCQ late treatment study: 18% shorter hospitalization (p=0.11).
Prospective observational study of 131 COVID-19 patients in Jordan, showing 18% shorter hospital stay with HCQ, p = 0.11. https://c19p.org/alqassieh
261. A. Desbois, C. Marques, L. Lefèvre, S. Barmo, C. Lorenzo, M. Leclercq, G. Leroux, C. Comarmond, C. Chapelon, F. Domont, M. Vautier, D. Saadoun, and P. Cacoub, Prevalence and clinical features of COVID-19 in a large cohort of 199 patients with sarcoidosis Jul 2020, Research Square
199 patient HCQ prophylaxis study: 17% fewer cases (p=1).
Retrospective 199 sarcoidosis patients showing non-statistically significant HCQ RR 0.83, p=1.0. https://c19p.org/desbois
262. M. Shabrawishi, A. Naser, H. Alwafi, A. Aldobyany, and A. Touman, Negative nasopharyngeal SARS-CoV-2 PCR conversion in response to different therapeutic interventions May 2020, medRxix
LATE TREATMENT 93 patient HCQ late treatment study: 15% improved viral clearance (p=0.66).
Retrospective 93 hospitalized patients in Saudi Arabia showing a non-statistically significant 15% reduction in PCR positive results at day 5, RR 0.85, p = 0.65. The treatment group had significantly more severe illness and significantly more male patients. https://c19p.org/shabrawishi
263. J. Chen, D. Liu, L. Liu, P. Liu, Q. Xu, L. Xia, Y. Ling, D. Huang, S. Song, D. Zhang, Z. Qian, T. Li, Y. Shen, H. Lu, A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) Mar 2020, J. Zhejiang University
LATE TREATMENT 30 patient HCQ late treatment RCT: 29% lower progression (p=0.57) and 100% worse viral clearance (p=1).
30 moderate hospitalized cases, all recovered. Time to RNA negative comparable. Less frequent radiological progression with HCQ but not statistically significant. One HCQ patient developed to a severe case. Treatment group 4 years older and with higher incidence of hypertension. https://c19p.org/chenmedsci
264. R. Sarhan, H. Harb, A. Abou Warda, M. Salem-Bekhit, F. Shakeel, S. Alzahrani, Y. Madney, and M. Boshra, Efficacy of the early treatment with tocilizumab-hydroxychloroquine and tocilizumab-remdesivir in severe COVID-19 Patients Nov 2021, J. Infection and Public Health, Volume 15, Issue 1, Page 116-122
LATE TREATMENT 108 patient HCQ late treatment RCT: 26% lower mortality (p=0.39), 26% higher hospital discharge (p=0.39), and 25% longer hospitalization (p=0.06).
Small 108 patient RCT comparing HCQ vs. remdesivir in very late stage treatment. All patients received tocilizumab. There were significant unadjusted baseline differences in ventilation and ICU admission. NCT04779047. https://c19p.org/sarhan
265. P. Salvador, P. Oliveira, T. Costa, M. Fidalgo, R. Neto, M. Silva, C. Figueiredo, V. Afreixo, T. Gregório, and L. Malheiro, Clinical Features and Prognostic Factors of 245 Portuguese Patients Hospitalized With COVID-19 Mar 2021, Cureus
LATE TREATMENT 245 patient HCQ late treatment study: 33% lower mortality (p=0.1), 448% higher ventilation (p=0.003), and 17% lower combined mortality/intubation (p=0.21).
Prospective study of 245 hospitalized patients, 121 treated with HCQ, showing lower (non-statistically significant) mortality and higher ventilation at 30 days. Confounding by indication is likely. https://c19p.org/salvador
266. M. Naseem, H. Arshad, S. Hashmi, F. Irfan, and F. Ahmed, Predicting mortality in SARS-COV-2 (COVID-19) positive patients in the inpatient setting using a Novel Deep Neural Network Dec 2020, medRxiv
LATE TREATMENT 1,214 patient HCQ late treatment study: 33% lower mortality (p=0.34).
Retrospective 1,214 hospitalized patients in Pakistan, 77 HCQ patients, showing 33% lower mortality with HCQ, multivariate Cox HR 0.67, p = 0.34. https://c19p.org/naseem
267. E. Afşin, Factors affecting prognosis and mortality in severe COVID-19 pneumonia patients Jul 2023, Acta Clinica Croatica
LATE TREATMENT 80 patient HCQ late treatment study: 17% lower mortality (p=0.5).
Retrospective 80 hospitalized severe COVID-19 patients in Turkey, showing no significant difference with HCQ treatment in unadjusted results. All patients received favipiravir. https://c19p.org/afsin
268. A. Shukla, S. Atal, A. Banerjee, R. Jhaj, S. Balakrishnan, P. Chugh, D. Xavier, A. Faruqui, A. Singh, R. Raveendran, J. Mathaiyan, J. Gauthaman, U. Parmar, R. Tripathi, S. Kamat, N. Trivedi, P. Shah, J. Chauhan, H. Dikshit, H. Mishra, R. Kumar, D. Badyal, M. Sharma, M. Singla, B. Medhi, A. Prakash, R. Joshi, N. Chatterjee, J. Cherian, V. Kamboj, and N. Kshirsagar, An observational multi-centric COVID-19 sequelae study among health care workers Dec 2022, The Lancet Regional Health – Southeast Asia, Volume 10, Page 100129
679 patient HCQ prophylaxis study: 5% lower PASC (p=0.78).
Retrospective 679 healthcare workers post COVID-19 discharge, 76 using HCQ prophylaxis, showing no significant difference in post-acute sequalae for covid. https://c19p.org/shukla
269. C. Hall, J. Jacobs, A. Stammers, J. St. Louis, J. Hayanga, M. Firstenberg, L. Mongero, E. Tesdahl, K. Rajagopal, F. Cheema, K. Patel, T. Coley, A. Sestokas, M. Slepian, and V. Badhwar, Multi-institutional Analysis of 505 COVID-19 Patients Supported with ECMO: Predictors of Survival Feb 2022, The Annals of Thoracic Surgery
LATE TREATMENT 505 patient HCQ ICU study: 11% lower mortality (p=0.31).
Retrospective extracorporeal membrane oxygenation (extremely high risk medical intervention) patients showing no significant difference in mortality in unadjusted results. https://c19p.org/hall
270. H. Alwafi, M. Shabrawishi, A. Naser, A. Aldobyany, S. Qanash, and A. Touman, Negative Nasopharyngeal SARS-CoV-2 PCR Conversion in Response to Different Therapeutic Interventions Jan 2022, Cureus
LATE TREATMENT 93 patient HCQ late treatment study: 15% improved viral clearance (p=0.65).
Retrospective 93 hospitalized patients in Saudi Arabia, 45 treated with CQ/HCQ, showing no significant difference in viral clearance. More patients treated with CQ/HCQ had severe cases at baseline (20% vs. 2%). https://c19p.org/alwafi
271. B. Tu, S. Lakoh, B. Xu, M. Lado, R. Cole, F. Chu, S. Hastings-Spaine, M. Jalloh, J. Zheng, W. Chen, and S. Sevalie, Risk Factors for Severity and Mortality in Adult Patients Confirmed with COVID-19 in Sierra Leone: A Retrospective Study Jan 2022, Infectious Diseases & Immunity, Volume 2, Issue 2, Page 83-92
LATE TREATMENT 180 patient HCQ late treatment study: 17% lower mortality (p=0.81).
Retrospective 180 hospitalized COVID-19 patients in Sierra Leone, showing no significant difference with HCQ treatment in unadjusted results, however HCQ was significantly more likely to be used for severe patients (33% vs. 12%). https://c19p.org/tu
272. M. Turrini, A. Gardellini, L. Beretta, L. Buzzi, S. Ferrario, S. Vasile, R. Clerici, A. Colzani, L. Liparulo, G. Scognamiglio, G. Imperiali, G. Corrado, A. Strada, M. Galletti, N. Castiglione, and C. Zanon, Clinical Course and Risk Factors for In-Hospital Mortality of 205 Patients with SARS-CoV-2 Pneumonia in Como, Lombardy Region, Italy Jun 2021, Vaccines, Volume 9, Issue 6, Page 640
LATE TREATMENT 205 patient HCQ late treatment study: 10% lower mortality (p=0.15).
Retrospective 205 patients in Italy, 160 treated with HCQ, showing lower mortality with treatment in multivariate analysis, but not reaching statistical significance. https://c19p.org/turrini
273. M. Haji Aghajani, O. Moradi, H. Amini, H. Azhdari Tehrani, E. Pourheidar, M. Rabiei, and M. Sistanizad, Decreased in-hospital mortality associated with aspirin administration in hospitalized patients due to severe COVID-19 Apr 2021, J. Medical Virology, Volume 93, Issue 9, Page 5390-5395
LATE TREATMENT 991 patient HCQ late treatment study: 19% lower mortality (p=0.09).
Retrospective 991 hospitalized patients in Iran, showing lower mortality with HCQ, not reaching statistical significance. https://c19p.org/hajiaghajani
274. M. Haji Aghajani, O. Moradi, H. Amini, H. Azhdari Tehrani, E. Pourheidar, M. Rabiei, and M. Sistanizad, Decreased In-Hospital Mortality Associated with Aspirin Administration in Hospitalized Patients Due to Severe COVID-19 Apr 2021, J. Medical Virology, Volume 93, Issue 9, Page 5390-5395
LATE TREATMENT 991 patient HCQ late treatment study: 19% lower mortality (p=0.09).
Retrospective 991 hospitalized patients in Iran focusing on aspirin use but also showing results for HCQ, remdesivir, and favipiravir. https://c19p.org/aghajani
275. K. Pham, H. Torres, M. Satlin, P. Goyal, and R. Gulick, Failure of chronic hydroxychloroquine in preventing severe complications of COVID-19 in patients with rheumatic diseases Mar 2021, Rheumatology Advances in Practice, Volume 5, Issue 1
42 patient HCQ prophylaxis study: 20% lower mortality (p=0.77) and 35% higher ICU admission (p=0.61).
Tiny retrospective database analysis of hospitalized COVID-19 patients with rheumatologic disease containing 14 chronic HCQ and 28 control patients. Patients are very poorly matched. Bias against HCQ is clear in the abstract which mentions differences favoring HCQ but ignores those favoring control (large differences in ethnicity, rheumatic conditions, hypertension, coronary artery disease, solid organ transplant recipients, immunosuppressive drugs). 61% of control patients also received HCQ (?). Adherence for chronic HCQ patients was not examined. Despite the very large differences between the groups, no adjustments are made. The study claims that HCQ did not prevent severe cases, but the study is among hospitalized patients, i.e., they already have cases severe enough for hospitalization – this study can not identify a protective effect of HCQ that reduces the probability of disease severe enough for hospitalization. https://c19p.org/pham
276. O. Ubaldo, J. Palo, and J. Cinco, COVID-19: A Single-Center ICU Experience of the First Wave in the Philippines Jan 2021, Critical Care Research and Practice, Volume 2021, Page 1-12
LATE TREATMENT 31 patient HCQ ICU study: 18% lower mortality (p=0.64).
Retrospective ICU patients in the Philippines showing unadjusted HCQ RR 0.82, p = 0.64. https://c19p.org/ubaldo
277. S. Ortonobes Roig, N. Soler-Blanco, I. Torrente Jiménez, E. Van den Eynde Otero, M. Moreno-Ariño, and M. Gómez-Valent, Clinical and pharmacological data in COVID-19 hospitalized nonagenarian patients Jan 2021, Revista Espanola de Quimioterapia, Volume 34, Issue 2, Page 145-150
LATE TREATMENT 79 patient HCQ late treatment study: 16% lower mortality (p=0.76).
Retrospective 79 hospitalized nonagenarian patients showing unadjusted HCQ mortality RR 0.84, p = 0.76. https://c19p.org/roig
278. M. Khoubnasabjafari, A. Jouyban, A. Malek Mahdavi, L. Namvar, K. Esalatmanesh, M. Hajialilo, S. Dastgiri, M. Soroush, S. Safiri, and A. Khabbazi, Prevalence of COVID-19 in patients with rheumatoid arthritis (RA) already treated with hydroxychloroquine (HCQ) compared with HCQ-naive patients with RA: a multicentre cross-sectional study Jan 2021, Postgraduate Medical J., Volume 98, Issue e2, Page e92-e93
1,858 patient HCQ prophylaxis study: 17% fewer cases (p=0.59).
Survey analysis of 1,858 RA patients in Iran, showing no significant difference in cases with HCQ prophylaxis. https://c19p.org/khoubnasabjafari
279. S. Tehrani, A. Killander, P. Åstrand, J. Jakobsson, and P. Gille-Johnson, Risk factors for mortality in adult COVID-19 patients: frailty predicts fatal outcome in older patients Oct 2020, Int. J. Infectious Diseases, Volume 102, Page 415-421
LATE TREATMENT 255 patient HCQ late treatment study: 13% lower mortality (p=0.63).
Retrospective 255 hospitalized patients, 65 treated with HCQ, showing unadjusted RR 0.87, p=0.63. Confounding by indication is likely. https://c19p.org/tehrani
280. Z. Pasquini, R. Montalti, C. Temperoni, B. Canovari, M. Mancini, M. Tempesta, D. Pimpini, N. Zallocco, and F. Barchiesi, Effectiveness of remdesivir in patients with COVID-19 under mechanical ventilation in an Italian ICU Aug 2020, J. Antimicrobial Chemotherapy, Volume 75, Issue 11, Page 3359-3365
LATE TREATMENT 51 patient HCQ ICU study: 16% lower mortality (p=0.34).
Retrospective 51 ICU patients under mechanical ventilation, 33 treated with HCQ, showing unadjusted lower mortality with treatment. https://c19p.org/pasquini
281. A. Ip, D. Berry, E. Hansen, A. Goy, A. Pecora, B. Sinclaire, U. Bednarz, M. Marafelias, S. Berry, N. Berry, S. Mathura, I. Sawczuk, N. Biran, R. Go, S. Sperber, J. Piwoz, B. Balani, C. Cicogna, R. Sebti, J. Zuckerman, K. Rose, L. Tank, L. Jacobs, J. Korcak, S. Timmapuri, J. Underwood, G. Sugalski, C. Barsky, D. Varga, A. Asif, J. Landolfi, and S. Goldberg, Hydroxychloroquine and Tocilizumab Therapy in COVID-19 Patients – An Observational Study May 2020, PLoS ONE, Volume 15, Issue 8, Page e0237693
LATE TREATMENT 2,512 patient HCQ late treatment study: 1% lower mortality (p=0.93).
Retrospective study of late stage use on 2,512 hospitalized patients showing no significant differences in associated mortality for patients receiving any HCQ during the hospitalization (HR, 0.99 [95% CI, 0.80-1.22]), HCQ alone (HR, 1.02 [95% CI, 0.83-1.27]), or HCQ+AZ (HR, 0.98 [95% CI, 0.75-1.28]). Misclassification is possible due to manual abstraction of EHR data. They observed a change in the prescribing patterns of HCQ during the study timeframe. Confounding by indication. https://c19p.org/ip2
282. S. Kamran, Z. Mirza, B. Naseem, F. Saeed, R. Azam, N. Ullah, W. Ahmad, and S. Saleem, Clearing the fog: Is HCQ effective in reducing COVID-19 progression: A randomized controlled trial Aug 2020, medRxiv
LATE TREATMENT 500 patient HCQ late treatment study: 5% lower progression (p=1) and 26% improved viral clearance (p=0.001).
Study of 349 low-risk hospitalized patients with 151 non-consenting or ineligible patients used as controls. Standard-of-care included zinc, vitamin C and vitamin D. A statistically significant improvement in PCR negativity is shown at day 7 with HCQ treatment, 52.1% (HCQ) versus 35.7% (control), p=0.001, but no statistically significant difference at day 14, or in progression. Patients were relatively young and there was no mortality. Only 3% of patients had any disease progression and all patients recovered, so there is little if any room for treatment benefit. Progression among higher-risk patients with comorbidities was lower with treatment (12.9% versus 28.6%, p=0.3, very few cases). Despite the title, this is not an RCT since patients self-selected the arm or were chosen based on allergies/contraindications. The treatment group had about twice the number of patients with comorbidities. Treatment delay is unknown – it was recorded but not reported in the paper. Viral load was not measured. As with other studies, PCR may detect non-replicable viral nucleic acid, this is more likely at day 14. Details on the test accuracy are not provided, authors note that RT-PCR sensitivity ranges from 34-80%. https://c19p.org/kamran
283. M. Barra, N. Carlos Medinacelli, C. Meza Padilla, L. Di Rocco, R. Larrea, G. Gaudenzi, V. Mastrovincenzo, E. Raña, I. Moreno, D. Sörvik, A. Sarlingo, F. Dadomo, and M. Torrilla, COVID-19 in hospitalized patients in 4 hospitals in San Isidro, Buenos Aires, Argentina Jul 2021, medRxiv
LATE TREATMENT 668 patient HCQ late treatment study: 11% lower mortality (p=1).
Retrospective 668 hospitalized patients in Argentina, 18 treated with HCQ, not showing a significant difference in unadjusted results. https://c19p.org/barra
284. M. An, M. Kim, Y. Park, B. Kim, S. Kang, W. Kim, S. Park, H. Park, W. Yang, J. Jang, S. Jang, and T. Hwang, Treatment Response to Hydroxychloroquine and Antibiotics for mild to moderate COVID-19: a retrospective cohort study from South Korea Jul 2020, medRxiv
LATE TREATMENT 226 patient HCQ late treatment study: 3% faster viral clearance (p=0.92).
Retrospective of hospitalized patients with 31 HCQ patients and 195 standard treatment patients, not showing a significant difference in terms of viral clearance or recovery. There was no mortality in either group. “It is notable that HQ plus antibiotics group had worse baseline clinical profiles (i.e. higher percentage of moderate severity patients, more patients with fever >=37.5C, higher average body temperature) and prognostic indicators such as age, LDH, lymphocyte count, and CRP.” We note that propensity score matching removed almost all of the male patients in the control group (40% to 5%) but increased the percentage of male patients in the treatment group. This provides a large advantage to the control group because there is a very large difference in severity and mortality based on gender. In terms of viral RNA clearance we note that other research has found that “active viral replication drops quickly after the first week, and viable virus was not found after the second week of illness despite the persistence of PCR detection of RNA” Viral load measured by PCR may not accurately reflect infectious virus measured by viral culture. Porter et al. show that viral load early in infection was correlated with infectious virus, but viral load late in infection could be high even with low or undetectable infectious virus. Assessing viral load later in infection may underestimate reductions in infectious virus with treatment. https://c19p.org/an
285. S. Singh, A. Khan, M. Chowdhry, and A. Chatterjee, Outcomes of Hydroxychloroquine Treatment Among Hospitalized COVID-19 Patients in the United States- Real-World Evidence From a Federated Electronic Medical Record Network May 2020, medRxiv
LATE TREATMENT 1,820 patient HCQ late treatment study: 5% lower mortality (p=0.72) and 19% lower ventilation (p=0.26).
EHR analysis of 3,372 hospitalized COVID-19 patients not showing a significant difference for mortality or the risk of mechanical ventilation. Subject to the limitations of EHR analysis. Misclassification is possible. Confounding by indication is likely. https://c19p.org/singh
286. J. Macias, P. Gonzalez-Moreno, E. Sanchez-Garcia, R. Morillo-Verdugo, C. Dominguez-Quesada, A. Pinilla, M. Macho, M. Martinez, A. Gonzalez-Serna, A. Corma, L. Real, and J. Pineda, Similar incidence of Coronavirus Disease 2019 (COVID-19) in patients with rheumatic diseases with and without hydroxychloroquine therapy May 2020, medRxiv
722 patient HCQ prophylaxis study: 26% lower hospitalization (p=1) and 49% more cases (p=0.53).
Very small retrospective study of rheumatic disease patients, sample size is too small for statistical significance (HCQ 0.5-4.0%, no-HCQ 0.4-2.7%). Confirmed cases were 1 HCQ and 2 no-HCQ, confirmed+likely cases were 1 HCQ and 3 no-HCQ. 1 HCQ and 2 no-HCQ patients were admitted to hospital. We do not think a conclusion can be drawn based on these sample sizes. There are very significant differences between the groups, for example 30% of the HCQ group have SLE vs. 2.5% of the no-HCQ group. SLE patients have a 5.7 times relative risk of pneumonia, whereas the relative risk with glucocorticoids and TNF-α inhibitors is significantly lower. Two more recent studies with rheumatic disease/autoimmune condition patients provide higher confidence. https://c19p.org/macias
287. E. Sbidian, J. Josse, G. Lemaitre, I. Meyer, M. Bernaux, A. Gramfort, N. Lapidus, N. Paris, A. Neuraz, I. Lerner, N. Garcelon, B. Rance, O. Grisel, T. Moreau, A. Bellamine, P. Wolkenstein, G. Varoquaux, E. Caumes, M. Lavielle, A. Dessap, and E. Audureau, Hydroxychloroquine with or without azithromycin and in-hospital mortality or discharge in patients hospitalized for COVID-19 infection: a cohort study of 4,642 in-patients in France Jun 2020, medRxiv
LATE TREATMENT 4,642 patient HCQ late treatment study: 5% higher mortality (p=0.74) and 20% higher hospital discharge (p=0.002).
Retrospective of 4,642 hospitalized patients in France showing significantly faster discharge with HCQ and HCQ+AZ. Patients receiving ‘HCQ alone’ or ‘HCQ plus AZI’ were more likely younger, males, current smokers and overall presented with slightly more co-morbidities (obesity, diabetes, any chronic pulmonary diseases, liver diseases). No significant effect is seen on 28-day mortality, however many more control patients are still in hospital at 28 days. Significantly higher rates of discharge home were observed in patients treated by HCQ. Other studies show faster resolution for HCQ, suggesting there will be a significant improvement when extending past 28 days. Note that the median age is higher in the group not treated with HCQ or AZ. For other issues with the adjustments see here. https://c19p.org/sbidian
288. A. Karruli, F. Boccia, M. Gagliardi, F. Patauner, M. Ursi, P. Sommese, R. De Rosa, P. Murino, G. Ruocco, A. Corcione, R. Andini, R. Zampino, and E. Durante-Mangoni, Multidrug-Resistant Infections and Outcome of Critically Ill Patients with Coronavirus Disease 2019: A Single Center Experience Aug 2021, Microbial Drug Resistance, Volume 27, Issue 9, Page 1167-1175
LATE TREATMENT 32 patient HCQ ICU study: 5% lower mortality (p=1).
Retrospective 32 ICU patients, showing no significant difference with HCQ treatment in unadjusted results. https://c19p.org/karrulih
289. M. Shabani, M. Totonchi, O. Rezaeimirghaed, L. Gachkar, M. Hajiesmaeili, A. Khoshkar, M. Amirdosara, A. Saffaei, S. Shokouhi, M. Mardani, I. Alavi Darazam, A. Karami, M. Sharifi, M. Zaman, E. Abedheydari, and Z. Sahraei, Evaluation of the Prophylactic Effect of Hydroxychloroquine on People in Close-Contact with Patients with Covid-19 Aug 2021, Pulmonary Pharmacology & Therapeutics, Volume 70, Page 102069
113 patient HCQ prophylaxis study: 19% fewer symptomatic cases (p=1) and 6% more cases (p=1).
Small PEP trial with 51 HCQ patients, not showing a significant difference in cases. IRCT20130917014693N10. https://c19p.org/shabani
290. C. Roger, O. Collange, M. Mezzarobba, O. Abou-Arab, L. Teule, M. Garnier, C. Hoffmann, L. Muller, J. Lefrant, P. Guinot, E. Novy, P. Abraham, T. Clavier, J. Bourenne, G. Besch, L. Favier, M. Fiani, A. Ouattara, O. Joannes-Boyau, M. Fischer, M. Leone, Y. Ait Tamlihat, J. Pottecher, P. Cordier, P. Aussant, M. Moussa, E. Hautin, M. Bouex, J. Julia, J. Cady, M. Danguy Des Déserts, N. Mayeur, T. Mura, and B. Allaouchiche, French Multicentre Observational Study on SARS-CoV-2 infections Intensive care initial management: the FRENCH CORONA Study Jul 2021, Anaesthesia Critical Care & Pain Medicine, Volume 40, Issue 4, Page 100931
LATE TREATMENT 966 patient HCQ ICU study: no change in mortality (p=0.94).
Prospective study of 966 ICU patients in France, 289 treated with HCQ, showing no significant difference with treatment. This study is excluded in the “after exclusion results” of meta analysis. Substantial confounding by time likely due to declining usage over the early stages of the pandemic when overall treatment protocols improved dramatically. https://c19p.org/roger
291. J. Jacobs, A. Stammers, J. St Louis, J. Hayanga, M. Firstenberg, L. Mongero, E. Tesdahl, K. Rajagopal, F. Cheema, K. Patel, T. Coley, A. Sestokas, M. Slepian, and V. Badhwar, Multi-institutional Analysis of 200 COVID-19 Patients treated with ECMO:Outcomes and Trends Jul 2021, The Annals of Thoracic Surgery, Volume 113, Issue 5, Page 1452-1460
LATE TREATMENT 200 patient HCQ late treatment study: 7% lower mortality (p=0.74).
Prospective study of 200 extracorporeal membrane oxygenation (extremely high risk medical intervention) patients showing no significant difference in unadjusted results for HCQ treatment. https://c19p.org/jacobs
292. F. Çiyiltepe, The Effect of Pre-admission Hydroxychloroquine Treatment on COVID-19-Related Intensive Care Follow-up in Geriatric Patients Apr 2021, South. Clin. Ist. Euras.
LATE TREATMENT 147 patient HCQ ICU study: 3% lower mortality (p=0.85).
Retrospective 147 ICU patients in Turkey, showing no significant difference in outcomes based on HCQ treatment before ICU admission. This study was not very informative, for example we do not know if HCQ treated patients were much less likely to be admitted to the ICU. https://c19p.org/ciyiltep
293. S. Spila Alegiani, S. Crisafulli, P. Giorgi Rossi, P. Mancuso, C. Salvarani, F. Atzeni, R. Gini, U. Kirchmayer, V. Belleudi, P. Kurotschka, O. Leoni, M. Ludergnani, E. Ferroni, S. Baracco, M. Massari, and G. Trifirò, Risk of COVID-19 hospitalization and mortality in rheumatic patients treated with hydroxychloroquine or other conventional DMARDs in Italy Apr 2021, Rheumatology, Volume 60, Issue SI, Page SI25-SI36
HCQ prophylaxis study: 8% higher mortality (p=0.64) and 18% lower hospitalization (p=0.03).
Retrospective database analysis case control study of rheumatic patients. When compared with other cDMARDs, HCQ users had significantly lower hospitalization, however there was no significant difference in mortality. Results differ significantly from previous studies, for example showing mortality OR 0.94 [0.83-1.06] for patients with rheumatic disease and mortality OR 0.88 [0.74-1.05] for patients with RA/SLE. Other research shows that the risk of COVID-19 for systemic autoimmune disease patients is much higher overall. https://c19p.org/alegiani
294. N. Vernaz, T. Agoritsas, A. Calmy, A. Gayet-Ageron, G. Gold, A. Perrier, F. Picard, V. Prendki, J. Reny, C. Samer, J. Stirnemann, P. Vetter, M. Zanella, D. Zekry, and S. Baggio, Early experimental COVID-19 therapies: associations with length of hospital stay, mortality and related costs Dec 2020, Swiss Medical Weekly, Volume 150, Issue 5153, Page w20446
LATE TREATMENT 198 patient HCQ late treatment propensity score matching study: 15% lower mortality (p=0.71) and 49% longer hospitalization (p=0.002).
Retrospective 840 hospitalized patients in Switzerland showing non-statistically significant lower mortality with HCQ but significantly longer hospitalization times. Confounding by indication is likely. Propensity score matching fails to adjust for severity with a 16% higher modified national early warning score for HCQ vs. standard-of-care in the matched cohort. Time varying confounding is likely. HCQ became controversial and was suspended towards the end of the period studied, therefore HCQ use was likely more frequent toward the beginning of the study period, a time when overall treatment protocols were significantly worse. Authors note: “overall, there was an indication bias, with the reason of prescription being associated with the outcome of interest. Indeed, patients with more severe COVID-19 were more likely to receive experimental therapies.” https://c19p.org/vernaz
295. F. Annie, C. Sirbu, K. Frazier, M. Broce, and B. Lucas, Hydroxychloroquine in hospitalized COVID-19 patients: Real world experience assessing mortality Oct 2020, Pharmacotherapy, Volume 40, Issue 11, Page 1072-1081
LATE TREATMENT 734 patient HCQ late treatment study: 4% lower mortality (p=0.83).
Retrospective database analysis with PSM not including COVID-19 severity, finding mortality OR 0.95 [0.62-1.46] for HCQ, and 1.24 [0.70-2.22] for HCQ+AZ. Confounding by indication likely. https://c19p.org/annie
296. F. Albani, F. Fusina, A. Giovannini, P. Ferretti, A. Granato, C. Prezioso, D. Divizia, A. Sabaini, M. Marri, E. Malpetti, and G. Natalini, Impact of Azithromycin and/or Hydroxychloroquine on Hospital Mortality in COVID-19 Aug 2020, J, Clinical Medicine, Volume 9, Issue 9, Page 2800
LATE TREATMENT 816 patient HCQ late treatment study: 18% lower mortality (p=0.15) and 9% higher ICU admission (p=0.7).
Retrospective 1376 hospitalized patients in Italy, 211 treated with HCQ and 166 with HCQ+AZ. https://c19p.org/albani
297. C. Salvarani, P. Mancuso, F. Gradellini, N. Viani, P. Pandolfi, M. Reta, G. Carrozzi, G. Sandri, G. Bajocchi, E. Galli, F. Muratore, L. Boiardi, N. Pipitone, G. Cassone, S. Croci, A. Marata, M. Costantini, and P. Giorgi Rossi, Susceptibility to COVID-19 in Patients Treated With Antimalarials: A Population-Based Study in Emilia-Romagna, Northern Italy Aug 2020, Arthritis & Rheumatology, Volume 73, Issue 1, Page 48-52
HCQ prophylaxis study: 6% fewer cases (p=0.75).
Comparison of CQ/HCQ users with the general population in a region of Italy, showing no significant difference in the probability of COVID-19. CQ/HCQ users were mostly systemic autoimmune disease patients and authors do not adjust for the very different baseline risk for these patients. Other research shows that the risk of COVID-19 for systemic autoimmune disease patients is much higher overall, Ferri et al. show OR 4.42, p<0.001. https://c19p.org/salvarani
298. Z. Gendebien, C. Von Frenckell, C. Ribbens, B. André, M. Thys, M. Gangolf, L. Seidel, M. Malaise, and O. Malaise, Systematic analysis of COVID-19 infection and symptoms in a systemic lupus erythematosus population: correlation with disease characteristics, hydroxychloroquine use and immunosuppressive treatments Jun 2020, Annals of the Rheumatic Diseases, Volume 80, Issue 6, Page e94-e94
225 patient HCQ prophylaxis study: 4% fewer cases (p=0.93).
Small study of SLE patients taking HCQ with a phone survey for COVID-19 suggestive symptoms. There was 2 hospitalizations (group not identified) and no ICU or death cases. A similar percentage of suspected infections were reported for HCQ users and non-HCQ users, RR 0.96, p = 0.93. Severity was not analyzed to determine if HCQ treated patients fared better. No adjustment for concomitant medications or severity of SLE. Only 5 cases were PCR confirmed. https://c19p.org/gendebien
299. M. Gianfrancesco, K. Hyrich, S. Al-Adely, L. Carmona, M. Danila, L. Gossec, Z. Izadi, L. Jacobsohn, P. Katz, S. Lawson-Tovey, E. Mateus, S. Rush, G. Schmajuk, J. Simard, A. Strangfeld, L. Trupin, K. Wysham, S. Bhana, W. Costello, R. Grainger, J. Hausmann, J. Liew, E. Sirotich, P. Sufka, Z. Wallace, J. Yazdany, P. Machado, and P. Robinson, Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry May 2020, Annals of the Rheumatic Diseases, 859-866, Volume 79, Issue 7, Page 859-866
600 patient HCQ prophylaxis study: 3% lower hospitalization (p=0.82).
Analysis of rheumatic disease patients showing no significant association between antimalarial therapy and hospitalization, OR=0.94 [0.57-1.57], p=0.82 after adjustments. https://c19p.org/gianfrancesco
300. M. Konig, A. Kim, M. Scheetz, E. Graef, J. Liew, J. Simard, P. Machado, M. Gianfrancesco, J. Yazdany, D. Langguth, and P. Robinson, Baseline use of hydroxychloroquine in systemic lupus erythematosus does not preclude SARS-CoV-2 infection and severe COVID-19 May 2020, Annals of the Rheumatic Diseases, Volume 79, Issue 10, Page 1386-1388
80 patient HCQ prophylaxis study: 3% lower hospitalization (p=0.88).
Analysis of 80 SLE patients diagnosed with COVID-19, showing the frequency of hospitalization did not differ between individuals using an antimalarial versus non-users (55% (16/29) vs 57% (29/51). https://c19p.org/konig
301. O. Gendelman, H. Amital, N. Bragazzi, A. Watad, and G. Chodick, Continuous Hydroxychloroquine or Colchicine Therapy Does Not Prevent Infection With SARS-CoV-2: Insights From a Large Healthcare Database Analysis May 2020, Autoimmunity Reviews, July 2020, Volume 19, Issue 7, Page 102566
14,520 patient HCQ prophylaxis study: 8% fewer cases (p=0.88).
Very small study of rheumatic disease/autoimmune disorder patients showing no significant difference but with only 3 chronic HCQ patient cases. Only considers people tested at a time when primarily symptomatic cases were tested. Other research shows that the risk of COVID-19 for systemic autoimmune disease patients is much higher overall, Ferri et al. show OR 4.42, p<0.001 which is the observed real-world risk, taking into account factors such as these patients potentially being more careful to avoid exposure. Adjusting for the difference in baseline risk using the result in Ferri et al. shows substantial benefit for HCQ, RR 0.211, but with only 3 HCQ cases the result is inconclusive. More recent studies with rheumatic disease/autoimmune condition patients provide higher confidence. https://c19p.org/gendelman
302. A. Rao, S. Veluswamy, B. Shankarappa, R. Reddy, N. Umesh, L. John, L. Mathew, and N. Shetty, Hydroxychloroquine as pre-exposure prophylaxis against COVID-19 infection among healthcare workers: a prospective cohort study Dec 2021, Expert Review of Anti-infective Therapy
1,294 patient HCQ prophylaxis study: 11% fewer cases (p=0.68).
Prospective PrEP study with low risk healthcare workers in India showing RR=0.89 [0.53-1.52]. There were no significant adverse effects. Only mean age and gender distribution are provided for baseline characteristics, no severity information is provided, and no adjustments were made. Authors analyze HCQ use for <8 vs. ≥8 weeks, noting a lack of statistical significance, but not providing the results. https://c19p.org/rao
303. K. Cortez, B. Demot, S. Bartolo, D. Feliciano, V. Ciriaco, I. Labi, D. Viray, J. Casuga, K. Camonayan-Flor, P. Gomez, M. Velasquez, T. Cajulao, J. Nigos, M. De Leon, D. Solimen, A. Go, F. Pizarro, L. Haya, R. Aswat, V. Mangati, C. Palaganas, M. Genuino, K. Cutiyog-Ubando, K. Tadeo, M. Longid, N. Catbagan, J. Bongotan, B. Dominguez-Villar, and J. Dalao, Clinical characteristics and outcomes of COVID-19 patients in a tertiary hospital in Baguio City, Philippines Nov 2021, Western Pacific Surveillance and Response J., Volume 12, Issue 4, Page 71-81
LATE TREATMENT 280 patient HCQ late treatment study: 15% lower mortality (p=1).
Retrospective 280 hospitalized patients in the Philippines, 25 treated with HCQ, not showing any significant differences in unadjusted results. https://c19p.org/cortez
304. K. Fitzgerald, C. Mecoli, M. Douglas, S. Harris, B. Aravidis, J. Albayda, E. Sotirchos, A. Hoke, A. Orbai, M. Petri, L. Christopher-Stine, A. Baer, J. Paik, B. Adler, E. Tiniakou, H. Timlin, P. Bhargava, S. Newsome, A. Venkatesan, V. Chaudhry, T. Lloyd, C. Pardo, B. Stern, M. Lazarev, B. Truta, S. Saidha, E. Chen, M. Sharp, N. Gilotra, E. Kasper, A. Gelber, C. Bingham, A. Shah, and E. Mowry, Risk Factors for Infection and Health Impacts of the COVID-19 Pandemic in People with Autoimmune Diseases Feb 2021, medRxiv
4,666 patient HCQ prophylaxis study: 9% fewer cases (p=0.54).
Retrospective 4666 people with autoimmune or inflammatory conditions, showing HCQ adjusted risk of COVID-19 OR 0.91 [0.68-1.23]. Results are not adjusted for the significantly different risk of COVID-19 depending on the type and severity of autoimmune or inflammatory condition. https://c19p.org/fitzgerald
305. A. Wang, X. Zhong, and Y. Hurd, Comorbidity and Sociodemographic determinants in COVID-19 Mortality in an US Urban Healthcare System Jun 2020, medRxiv
LATE TREATMENT 7,592 patient HCQ late treatment study: 6% lower mortality (p=0.63).
Database analysis of 7,592 patients in NYC, showing adjusted HCQ mortality odds ratio OR 0.96, p = 0.82, and HCQ+AZ OR 0.94, p = 0.63 https://c19p.org/wangrx
306. E. Lamback, M. Oliveira, A. Haddad, A. Vieira, A. Neto, T. Maia, J. Chrisman, P. Spineti, M. Mattos, and E. Costa, Hydroxychloroquine with azithromycin in patients hospitalized for mild and moderate COVID-19 Feb 2021, The Brazilian J. Infectious Diseases, Volume 25, Issue 2, Page 101549
LATE TREATMENT 193 patient HCQ late treatment study: 9% lower mortality (p=0.83), 20% higher ICU admission (p=0.61), and 12% shorter hospitalization.
Retrospective 193 hospitalized patients in Brazil not finding a significant difference with HCQ. The control group was composed of patients refusing HCQ or with contraindications. Time based confounding is very likely because HCQ became more controversial in Brazil over the time covered (Mar – Jun 2020), while overall treatment protocols during this period improved dramatically, i.e., more control patients (those refusing HCQ) likely come later in the period when treatment protocols were greatly improved. The paper does not mention the word “confounding” or make any adjustments. https://c19p.org/lamback
307. S. Roy, S. Samajdar, S. Tripathi, S. Mukherjee, and K. Bhattacharjee, Outcome of Different Therapeutic Interventions in Mild COVID-19 Patients in a Single OPD Clinic of West Bengal: A Retrospective study Mar 2021, medRxiv
EARLY TREATMENT 29 patient HCQ early treatment study: 2% faster recovery (p=0.96).
Retrospective database analysis of 56 mild COVID-19 patients, all treated with vitamin C, vitamin D, and zinc, comparing ivermectin + doxycycline (n=14), AZ (n=13), HCQ (n=14), and standard-of-care (n=15), finding that all groups recover quickly, and there was no significant difference between the groups. https://c19p.org/royh
308. Xia et al., Efficacy of Chloroquine and Lopinavir/ Ritonavir in mild/general novel coronavirus (CoVID-19) infections: a prospective, open-label, multicenter randomized controlled clinical study Feb 2020, ChiCTR2000029741
LATE TREATMENT 25 patient HCQ late treatment study: 38% improved viral clearance (p=0.17).
Early results from a very small trial, reported within the application for a later trial. Very minimal details are provided, but we include this as the earliest published results. For COVID-19 patients with pneumonia the viral negative conversion rate was 50% (5/10) with CQ versus 20% (3/15) with lopinavir/ritonavir. https://c19p.org/xia
309. O. Küçükakkaş and T. Aydın, The effect of hydroxychloroquine against SARS-CoV-2 infection in rheumatoid arthritis patients Jul 2021, Research Square
17 patient HCQ prophylaxis study: 43% higher ICU admission (p=1).
Retrospective 17 rheumatoid arthritis COVID-19+ patients, 7 on HCQ treatment, showing no significant differences. They study reports only including hospitalized patients, but the results include non-hospitalized patients. Results do not reflect potential difference in the probability that a case is serious enough to have been tested and identified. Few group details are provided (even the age of patients in each group is not specified). https://c19p.org/kucukakkas
310. M. Salehi, M. Mohammadi, S. Abtahi, S. Ghazi, A. Sobati, R. Bozorgmehr, S. Manshadi, S. Siahkali, M. Mohammadi, B. Badie, and E. Rahimi, Risk factors of death in mechanically ventilated COVID-19 patients: a retrospective multi-center study Mar 2022, Research Square
LATE TREATMENT 125 patient HCQ ICU study: 14% higher mortality (p=0.44).
Retrospective 125 mechanically ventilated ICU patients in Iran, showing no significant difference with HCQ treatment in unadjusted results. https://c19p.org/salehih
311. F. Alhamlan, R. Almaghrabi, E. Devol, A. Alotaibi, S. Alageel, D. Obeid, B. Alraddadi, S. Althawadi, M. Mutabagani, and A. Al-Qahtani, Epidemiology and Clinical Characteristics in Individuals with Confirmed SARS-CoV-2 Infection During the Early COVID-19 Pandemic in Saudi Arabia Jul 2021, medRxiv
LATE TREATMENT HCQ late treatment study: 52% higher mortality (p=0.58).
Retrospective hospitalized patients in Saudi Arabia showing higher mortality with most treatments although not reaching statistical significance. Confounding by indication, time, or other factors is likely (a 19x higher risk with lopinavir/ritonavir and 3.5x higher risk with azithromycin is not supported by other studies for example). The number of patients treated with HCQ is not provided. https://c19p.org/alhamlan
312. S. Sarfaraz, Q. Shaikh, S. Saleem, A. Rahim, F. Herekar, S. Junejo, and A. Hussain, Determinants of in-hospital mortality in COVID-19; a prospective cohort study from Pakistan Jan 2021, medRxiv
LATE TREATMENT 186 patient HCQ late treatment study: 45% higher mortality (p=0.07).
Retrospective 186 hospitalized patients in Pakistan showing unadjusted HCQ mortality RR 1.45, p = 0.07. Confounding by indication is likely. https://c19p.org/sarfaraz
313. A. Abdulrahman, I. AlSayed, M. AlMadhi, J. AlArayed, S. Mohammed, A. Sharif, K. Alansari, A. AlAwadhi, and M. AlQahtani, The efficacy and safety of hydroxychloroquine in COVID19 patients : a multicenter national retrospective cohort Nov 2020, medRxiv
LATE TREATMENT 446 patient HCQ late treatment propensity matched scoring study: 17% lower mortality (p=1) and 75% higher combined mortality/intubation (p=0.24).
Retrospective analysis of acute care patients in Bahrain not showing a significant effect of HCQ. Confounding by indication is likely. Matching appears not to have matched for baseline severity. 17.5% of HCQ patients required oxygen while only 12.6% of control patients did. https://c19p.org/abdulrahman
314. F. Ader, N. Peiffer-Smadja, J. Poissy, M. Bouscambert-Duchamp, D. Belhadi, A. Diallo, C. Delmas, J. Saillard, A. Dechanet, N. Mercier, A. Dupont, T. Alfaiate, F. Lescure, F. Raffi, F. Goehringer, A. Kimmoun, S. Jaureguiberry, J. Reignier, S. Nseir, F. Danion, R. Clere-Jehl, K. Bouiller, J. Navellou, V. Tolsma, A. Cabie, C. Dubost, J. Courjon, S. Leroy, J. Mootien, R. Gaci, B. Mourvillier, E. Faure, V. Pourcher, S. Gallien, O. Launay, K. Lacombe, J. Lanoix, A. Makinson, G. Martin-Blondel, L. Bouadma, E. Botelho-Nevers, A. Gagneux-Brunon, O. Epaulard, L. Piroth, F. Wallet, J. Richard, J. Reuter, T. Staub, B. Lina, M. Noret et al., An open-label randomized, controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-beta-1a and hydroxychloroquine in hospitalized patients with COVID-19 – Final results from the DisCoVeRy trial Oct 2020, medRxiv
LATE TREATMENT 299 patient HCQ late treatment RCT: 15% higher mortality (p=0.7) and 24% improved viral clearance (p=0.68).
Early terminated very late stage (95% on oxygen at baseline) DisCoVeRy trial. 4% more patients were on ventilation at baseline in the HCQ group. This preprint presents more recent results than the earlier journal article. https://c19p.org/discovery
315. F. Shamsi, M. Karimi, Z. Nafei, and E. Akbarian, Survival and Mortality in Hospitalized Children with COVID-19: A Referral Center Experience in Yazd, Iran Jul 2023, Canadian J. Infectious Diseases and Medical Microbiology, Volume 2023, Page 1-12
LATE TREATMENT 183 patient HCQ late treatment study: 39% higher mortality (p=0.51).
Retrospective 183 hospitalized pediatric COVID-19 patients in Iran, showing no significant difference in mortality with in unadjusted results. https://c19p.org/shamsih
316. P. Kamstrup, P. Sivapalan, J. Eklöf, N. Hoyer, C. Ulrik, L. Pedersen, T. Lapperre, Z. Harboe, U. Bodtger, R. Bojesen, K. Håkansson, C. Tidemandsen, K. Armbruster, A. Browatzki, H. Meteran, C. Meyer, K. Skaarup, M. Lassen, J. Lundgren, T. Biering-Sørensen, and J. Jensen, Hydroxychloroquine as a primary prophylactic agent against sars-cov-2 infection: a cohort study May 2021, Int. J. Infectious Diseases, Volume 108, Page 370-376
60,334 patient HCQ prophylaxis study: 44% higher hospitalization (p=0.25) and 10% fewer cases (p=0.23).
Retrospective HCQ users in Denmark, not showing a significant difference, however authors do not adjust for the very different baseline risk for systemic autoimmune disease patients. Authors appear unaware of research in the area, for example saying that “currently, no obvious connection exists between a known rheumatological disorder and the risk of contracting SARS-CoV-2.” Many papers show that the risk of COVID-19 for systemic autoimmune disease patients is much higher overall, e.g., Ferri et al. show OR 4.42, p<0.001. Supplementary data is not available. https://c19p.org/kamstrup
317. A. El-Solh, U. Meduri, Y. Lawson, M. Carter, and K. Mergenhagen, Clinical course and outcome of COVID-19 acute respiratory distress syndrome: data from a national repository Oct 2020, medRxiv
LATE TREATMENT 643 patient HCQ late treatment study: 18% higher mortality (p=0.17).
Retrospective database analysis of 7,816 Veterans Affairs hospitalized patients analyzing progression to acute respiratory distress syndrome and 30-day mortality from acute respiratory distress syndrome. Confounding by indication is likely. Chronological bias is likely, with HCQ more likely to be used earlier on, before significant improvements in overall treatment. No results are provided for HCQ for progression to acute respiratory distress syndrome. https://c19p.org/solh
318. S. Saleemi, A. Alrajhi, M. Alhajji, A. Alfattani, and F. Albaiz, Time to negative PCR from symptom onset in COVID-19 patients on Hydroxychloroquine and Azithromycin – A real world experience Aug 2020, medRxiv
LATE TREATMENT 85 patient HCQ late treatment study: 21% slower viral clearance (p=0.05).
Retrospective 65 HCQ+AZ, 20 control patients, showing median time to negative PCR of 23 days for HCQ+AZ vs. 19 days for control. Confounding by indication. 100% of non-HCQ group had mild disease vs. 63% of the HCQ+AZ group. More comorbidities and symptoms in the HCQ+AZ group. https://c19p.org/saleemi
319. B. Alosaimi, H. Alshanbari, M. Alturaiqy, H. AlRawi, S. Alamri, A. Albujaidy, A. Bin Sabaan, A. Alrashed, A. Alamer, F. Alghofaili, K. Al-Duraymih, A. Alshalani, and W. Alturaiki, Analyzing the Difference in the Length of Stay (LOS) in Moderate to Severe COVID-19 Patients Receiving Hydroxychloroquine or Favipiravir Nov 2022, Pharmaceuticals, Volume 15, Issue 12, Page 1456
LATE TREATMENT 74 patient HCQ late treatment PSM study: 400% higher mortality (p=0.49), 43% shorter hospitalization (p=0.63), and 29% higher hospital discharge (p=0.74).
Retrospective 200 hospitalized COVID-19 patients in Saudi Arabia, showing no significant difference in outcomes between HCQ and favipiravir. https://c19p.org/alosaimi
320. A. Lyashchenko, Y. Yu, D. McMahon, R. Bies, M. Yin, and S. Cremers, Systemic Exposure to Hydroxychloroquine and its relationship with outcome in severely ill COVID-19 patients in New York City Aug 2022, British J. Clinical Pharmacology
LATE TREATMENT 3,256 patient HCQ late treatment study: 48% higher mortality (p<0.0001).
Retrospective very late stage hospitalized patients in New York during the first wave, showing no significant relationship between HCQ levels and outcomes. Authors note that the patients with data were the sickest patients. https://c19p.org/lyashchenko
321. A. Malundo, C. Abad, M. Salamat, J. Sandejas, J. Poblete, J. Planta, S. Morales, R. Gabunada, A. Evasan, J. Cañal, J. Santos, J. Manto, M. Mercado, R. Rojo, E. Ornos, and M. Alejandria, Predictors of Mortality among inpatients with COVID-19 Infection in a Tertiary Referral Center in the Philippines Jul 2022, IJID Regions
LATE TREATMENT 1,215 patient HCQ late treatment study: 24% higher mortality (p=0.32).
Retrospective 1,215 hospitalized patients in the Philippines, showing no significant difference in outcomes with remdesivir or HCQ use in unadjusted results subject to confounding by indication. https://c19p.org/malundo
322. A. Soto, D. Quiñones-Laveriano, J. Azañero, R. Chumpitaz, J. Claros, L. Salazar, O. Rosales, L. Nuñez, D. Roca, and A. Alcantara, Mortality and associated risk factors in patients hospitalized due to COVID-19 in a Peruvian reference hospital Mar 2022, PLOS ONE, Volume 17, Issue 3, Page e0264789
LATE TREATMENT 1,418 patient HCQ late treatment study: 6% higher mortality (p=0.46).
Retrospective 1,418 very late stage (46% mortality) patients in Peru, showing no significant difference with HCQ. There is strong confounding by indication, for example 48% of patients with baseline SpO2 <70% were treated compared with 22% for SpO2 >95%. There may also be significant confounding by time with standard-of-care changing substantially over the first few months of the pandemic. https://c19p.org/sotoh
323. M. Albanghali, S. Alghamdi, M. Alzahrani, B. Barakat, A. Haseeb, J. Malik, S. Ahmed, and S. Anwar, Clinical Characteristics and Treatment Outcomes of Mild to Moderate Covid-19 Patients in Saudi Arabia: A Single Centre Study Feb 2022, J. Infection and Public Health, Volume 15, Issue 3, Page 331-337
LATE TREATMENT 811 patient HCQ late treatment study: 35% higher mortality (p=0.46).
Retrospective 811 hospitalized COVID+ patients in Saudi Arabia, showing higher mortality with HCQ treatment in unadjusted results subject to confounding by indication. https://c19p.org/albanghali
324. S. Alghamdi, Clinical characteristics and treatment outcomes of severe (ICU) COVID-19 patients in Saudi Arabia: A single centre study Aug 2021, Saudi Pharmaceutical J., Volume 29, Issue 10, Page 1096-1101
LATE TREATMENT 171 patient HCQ ICU study: 39% higher mortality (p=0.52).
Retrospective 171 ICU patients in Saudi Arabia showing no significant difference for HCQ treatment in unadjusted results. https://c19p.org/alghamdi2
325. K. Gadhiya, P. Hansrivijit, M. Gangireddy, and J. Goldman, Clinical characteristics of hospitalised patients with COVID-19 and the impact on mortality: a single-network, retrospective cohort study from Pennsylvania state Apr 2021, BMJ Open, Volume 11, Issue 4, Page e042549
LATE TREATMENT 271 patient HCQ late treatment study: 5% higher mortality (p=0.89).
Retrospective 283 patients in the USA showing higher mortality with all treatments (not statistically significant). Confounding by indication is likely. In the supplementary appendix, authors note that the treatments were usually given for patients that required oxygen therapy. Oxygen therapy and ICU admission (possibly, the paper includes ICU admission for model 2 in some places but not others) were the only variables indicating severity used in adjustments. Time based confounding is likely because HCQ became increasingly controversial and less used over the time covered (March 1 to May 31, 2020), while overall treatment protocols during this period improved dramatically, i.e., more control patients likely come later in the period when treatment protocols were greatly improved. https://c19p.org/gadhiya
326. E. Mulhem, A. Oleszkowicz, and D. Lick, 3219 hospitalised patients with COVID-19 in Southeast Michigan: a retrospective case cohort study Apr 2021, BMJ Open, Volume 11, Issue 4, Page e042042
LATE TREATMENT 3,219 patient HCQ late treatment study: 28% higher mortality (p=0.1).
Retrospective database analysis of 3,219 hospitalized patients in the USA. Very different results in the time period analysis (Table S2), and results significantly different to other studies for the same medications (e.g., heparin OR 3.06 [2.44-3.83]) suggest significant confounding by indication and confounding by time. https://c19p.org/mulhem
327. S. Alghamdi, B. Barakat, I. Berrou, A. Alzahrani, A. Haseeb, M. Hammad, S. Anwar, A. Sindi, H. Almasmoum, and M. Albanghali, Clinical Efficacy of Hydroxychloroquine in Patients with COVID-19: Findings from an Observational Comparative Study in Saudi Arabia Mar 2021, Antibiotics, Volume 10, Issue 4, Page 365
LATE TREATMENT 775 patient HCQ late treatment study: 7% higher mortality (p=0.88).
Retrospective 775 hospitalized patients in Saudi Arabia showing no significant difference. There was no adjustment for severity or comorbidities. Confounding by indication is likely. https://c19p.org/alghamdi
328. N. Rosenthal, Z. Cao, J. Gundrum, J. Sianis, and S. Safo, Risk Factors Associated With In-Hospital Mortality in a US National Sample of Patients With COVID-19 Dec 2020, JAMA Network Open, Volume 3, Issue 12, Page e2029058
LATE TREATMENT HCQ late treatment study: 8% higher mortality (p=0.13).
Retrospective database analysis of 64,781 hospitalized patients in the USA, showing lower mortality with vitamin C or vitamin D (authors do not distinguish between the two), and higher mortality with zinc and HCQ, statistically significant for zinc. Authors excluded hospital-based outpatient visits, without explanation. Confounding by indication is likely, adjustments do not appear to include any information on COVID-19 severity at baseline. https://c19p.org/rosenthal
329. S. Aboulenain, N. Dewaswala, F. Ramos, P. Torres, A. Abdallah, M. Abdul Qader, B. Al-Abbasi, C. Bornmann, K. Dziadkowiec, K. Chen, J. Pino, R. Chait, and K. De Almeida, The Effect of Hydroxychloroquine on In-Hospital Mortality in COVID-19 Nov 2020, HCA Healthcare J. Medicine, Volume 1, Issue 0
LATE TREATMENT 175 patient HCQ late treatment study: 15% higher mortality (p=0.72).
Retrospective 175 hospitalized COVID-19 patients in the USA, showing no significant difference in mortality with HCQ. Authors note that “patients treated with HCQ in our cohort were more likely to be sicker at baseline.” https://c19p.org/aboulenain
330. G. Rodriguez-Nava, M. Yanez-Bello, D. Trelles-Garcia, C. Chung, S. Chaudry, A. Khan, H. Friedman, and D. Hines, Clinical characteristics and risk factors for mortality of hospitalized patients with COVID-19 in a community hospital: A retrospective cohort study Nov 2020, Mayo Clinic Proceedings: Innovations, Quality & Outcomes, Volume 5, Issue 1, Page 1-10
LATE TREATMENT 313 patient HCQ late treatment study: 6% higher mortality (p=0.77).
Retrospective 313 patients, mostly critical stage and mostly requiring respiratory support, showing unadjusted RR 1.06, p = 0.77. Confounding by indication likely. https://c19p.org/rodrigueznava
331. E. Salazar, P. Christensen, E. Graviss, D. Nguyen, B. Castillo, J. Chen, B. Lopez, T. Eagar, X. Yi, P. Zhao, J. Rogers, A. Shehabeldin, D. Joseph, F. Masud, C. Leveque, R. Olsen, D. Bernard, J. Gollihar, and J. Musser, Significantly Decreased Mortality in a Large Cohort of Coronavirus Disease 2019 (COVID-19) Patients Transfused Early with Convalescent Plasma Containing High-Titer Anti–Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Protein IgG Nov 2020, The American J. Pathology, Volume 191, Issue 1, Page 90-107
LATE TREATMENT 903 patient HCQ late treatment study: 37% higher mortality (p=0.28).
Convalescent plasma study also showing mortality based on HCQ treatment, unadjusted hazard ratio uHR 1.37, p = 0.28. Confounding by indication is likely. https://c19p.org/salazar
332. M. Choi, M. Kang, S. Shin, J. Noh, H. Cheong, W. Kim, J. Jung, and J. Song, Comparison of antiviral effect for mild-to-moderate COVID-19 cases between lopinavir/ritonavir versus hydroxychloroquine: A nationwide propensity score-matched cohort study Oct 2020, Int. J. Infectious Diseases, Volume 102, Page 275-281
LATE TREATMENT 1,402 patient HCQ late treatment study: 22% slower viral clearance (p=0.0001).
Health insurance database analysis failing to adjust for disease severity and not finding a significant difference in time to PCR- for LPV/r and HCQ. There are large differences in severity across groups. Authors did propensity score matching but chose not to prioritize severity, resulting in incomparable groups, e.g., baseline pneumonia of 44% in the HCQ group and 15% in the control group (after PSM). Authors note this but offer no explanation for not correcting for severity: “However, the disease severity and proportion of accompanying pneumonia were still significantly higher in the LPV/r and HCQ-group.” https://c19p.org/choi
333. C. Rentsch, N. DeVito, B. MacKenna, C. Morton, K. Bhaskaran, J. Brown, A. Schultze, W. Hulme, R. Croker, A. Walker, E. Williamson, C. Bates, S. Bacon, A. Mehrkar, H. Curtis, D. Evans, K. Wing, P. Inglesby, R. Mathur, H. Drysdale, A. Wong, H. McDonald, J. Cockburn, H. Forbes, J. Parry, F. Hester, S. Harper, L. Smeeth, I. Douglas, W. Dixon, S. Evans, L. Tomlinson, and B. Goldacre, Effect of pre-exposure use of hydroxychloroquine on COVID-19 mortality: a population-based cohort study in patients with rheumatoid arthritis or systemic lupus erythematosus using the OpenSAFELY platform Sep 2020, The Lancet Rheumatology, Volume 3, Issue 1, Page e19-e27
194,637 patient HCQ prophylaxis study: 3% higher mortality (p=0.83).
Observational database study of RA/SLE patients in the UK, 194,637 RA/SLE patients with 30,569 having >= 2 HCQ prescriptions in the prior 6 months, HCQ HR 1.03 [0.80-1.33] (HR 0.78 before adjustments). 70 patients with HCQ prescriptions died. One major problem is that there is no knowledge of compliance for these 70 patients for example, it is possible that they were part of the expected percentage of patients that did not take the medication as prescribed, invalidating the result. Other limitations include confounding by use of bDMARDs and confounding by severity of rheumatological disease. https://c19p.org/rentsch
334. M. Fried, J. Crawford, A. Mospan, S. Watkins, B. Munoz, R. Zink, S. Elliott, K. Burleson, C. Landis, K. Reddy, and R. Brown, Patient Characteristics and Outcomes of 11,721 Patients with COVID19 Hospitalized Across the United States Aug 2020, Clinical Infectious Disease, Volume 72, Issue 10, Page e558-e565
LATE TREATMENT 11,721 patient HCQ late treatment study: 27% higher mortality (p=0.001).
Database analysis of 11,721 hospitalized patients, 4,232 on HCQ. Strong evidence for confounding by indication and compassionate use of HCQ. 24.9% of HCQ patients were on mechanical ventilation versus 12.2% control. Ventilation mortality was 70.5% versus 11.6%. This study does not adjust for the differences in comorbid conditions and disease severity, and therefore does not make a conclusion. Unadjusted HCQ mortality was 24.8% versus control 19.6%. Adjusting for ventilation only gives us 17.7% HCQ versus 19.6% control (adjusting the HCQ group to have the same proportion of ventilation patients), RR 0.90. Hopefully authors can do a full adjustment analysis. Comorbidities may favor control, while patients remaining in the hospital (5.3%) may favor HCQ (other studies show faster resolution for HCQ patients). Data inconsistencies have been found in this study, for example 99.4% of patients treated with HCQ were treated in urban hospitals, compared to 65% of untreated patients (Supplemental Table 3), while patients are distributed in a more balanced manner between teaching or not-teaching hospitals, as well as in the most urbanized (Northeast) and less urbanized (Midwest) regions of the United States. https://c19p.org/fried
335. E. Peters, D. Collard, S. Van Assen, M. Beudel, M. Bomers, J. Buijs, L. De Haan, W. De Ruijter, R. Douma, P. Elbers, A. Goorhuis, N. Gritters van den Oever, L. Knarren, H. Moeniralam, R. Mostard, M. Quanjel, A. Reidinga, R. Renckens, J. Van Den Bergh, I. Vlasveld, and J. Sikkens, Outcomes of Persons With COVID-19 in Hospitals With and Without Standard Treatment With (Hydroxy)chloroquine Aug 2020, Clinical Microbiology and Infection, Volume 27, Issue 2, Page 264-268
LATE TREATMENT 1,949 patient HCQ late treatment study: 9% higher mortality (p=0.57).
Retrospective study of HCQ use in 9 hospitals in the Netherlands, showing no significant difference in mortality with HCQ/CQ or dexamethasone. Late stage (admitted to hospital with positive test or CT scan abnormalities). 4 of 7 hospitals started treatment only after further deterioration. Short cutoff (21 days) – other studies have shown treated patient cases resolved faster and more control patients remaining in hospital at this time. In the preprint, 58 of 341 control patients died. In the journal version, 53 of 353 control patients died. Significant differences between hospitals – HCQ hospitals had significantly older patients with significantly more comorbidities. Non-HCQ hospitals were “tertiary academic centers” whereas HCQ hospitals were “secondary care hospitals.” Residual confounding likely. This study compares overcrowded regular hospitals with undercrowded academic hospitals. A subset of patients were excluded due to transfer to other hospitals. This introduces bias because patients in critical condition are not transferred. For examples, patients benefiting from HCQ treatment may have been transferred to the tertiary centers and excluded from analysis, increasing the percentage of critical cases in the secondary hospitals. Among the seven CQ/HCQ hospitals, the timing of start of CQ/HCQ treatment differed; three hospitals started at the moment of COVID-19 diagnosis, four started after diagnosis but only when patients clinically deteriorated e.g., when there was an increase in respiratory rate or increase in use of supplemental oxygen. Most patients received CQ instead of the safer HCQ, receiving late treatment with CQ. Patients were given an initial dose of 600mg CQ then every 12 hours, for 5 days a dose of 300 mg, for a total of 3600mg CQ, likely to be toxic. Authors mention a subset of hospitals started treatment relatively earlier, which seems like the most important area to analyze, but no results are provided. https://c19p.org/peters
336. S. Roomi, W. Ullah, F. Ahmed, S. Farooq, U. Sadiq, A. Chohan, M. Jafar, M. Saddique, S. Khanal, R. Watson, and M. Boigon, Efficacy of hydroxychloroquine and tocilizumab in patients with COVID-19: A single-center retrospective chart review Aug 2020, J. Medical Internet Research, Volume 22, Issue 9, Page e21758
LATE TREATMENT 176 patient HCQ late treatment study: 38% higher mortality (p=0.54).
Retrospective 176 hospitalized patients (144 HCQ, 32 control) showing no significant differences with HCQ or TCZ. Confounding by indication. https://c19p.org/roomi
337. M. Singer, D. Kaelber, and M. Antonelli, Hydroxychloroquine ineffective for COVID-19 prophylaxis in lupus and rheumatoid arthritis Aug 2020, Annals of the Rheumatic Diseases, Volume 81, Issue 9, Page e161-e161
32,758 patient HCQ prophylaxis study: 9% more cases (p=0.62).
Comparison of the percentage of SLE/RA patients on immunosuppressants that were taking HCQ, for COVID-19 diagnosis versus other infections or outpatient visits, finding a similar percentage in each case. No mortality of severity information is provided to determine if HCQ treated patients fared better. No adjustment for concomitant medications or severity. https://c19p.org/singer
338. S. Gupta, S. Hayek, W. Wang, L. Chan, K. Mathews, M. Melamed, S. Brenner, A. Leonberg-Yoo, E. Schenck, J. Radbel, J. Reiser, A. Bansal, A. Srivastava, Y. Zhou, A. Sutherland, A. Green, A. Shehata, N. Goyal, A. Vijayan, J. Velez, S. Shaefi, C. Parikh, J. Arunthamakun, A. Athavale, A. Friedman, S. Short, Z. Kibbelaar, S. Abu Omar, A. Admon, J. Donnelly, H. Gershengorn, M. Hernán, M. Semler, D. Leaf, C. Walther, S. Anumudu, K. Kopecky, G. Milligan, P. McCullough, T. Nguyen, M. Krajewski, S. Shankar, A. Pannu, J. Valencia, S. Waikar, P. Hart, O. Ajiboye, M. Itteera, J. Rachoin, C. Schorr et al., Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US Jul 2020, JAMA Intern. Med., Volume 180, Issue 11, Page 1436
LATE TREATMENT 2,215 patient HCQ late treatment study: 6% higher mortality (p=0.41).
Analysis of 2,215 intensive care unit patients showing no significant differences with this very late stage use of HCQ. https://c19p.org/gupta
339. J. Sosa-García, A. Gutiérrez-Villaseñor, A. García-Briones, J. Romero-González, E. Juárez-Hernández, and O. González-Chon, Experience in the management of severe COVID-19 patients in an intensive care unit Jun 2020, Cir Cir. 2020, 569-575, Volume 88, Issue 5
LATE TREATMENT 56 patient HCQ ICU study: 11% higher mortality (p=1).
Small retrospective study of 56 ICU patients in Mexico showing HCQ RR 1.1, p = 1.0. https://c19p.org/sosagarcia
340. J. Luo, H. Rizvi, I. Preeshagul, J. Egger, D. Hoyos, C. Bandlamudi, C. McCarthy, C. Falcon, A. Schoenfeld, K. Arbour, J. Chaft, R. Daly, A. Drilon, J. Eng, A. Iqbal, W. Lai, B. Li, P. Lito, A. Namakydoust, K. Ng, M. Offin, P. Paik, G. Riely, C. Rudin, H. Yu, M. Zauderer, M. Donoghue, M. Łuksza, B. Greenbaum, M. Kris, and M. Hellmann, COVID-19 in patients with lung cancer Jun 2020, Annals of Oncology, 1386-1396, Volume 31, Issue 10, Page 1386-1396
LATE TREATMENT 48 patient HCQ late treatment study: 2% higher mortality (p=0.99).
Analysis of hospitalized lung cancer patients with 35 of 48 taking HCQ, mortality OR 1.03, p = 0.99. https://c19p.org/luo
341. E. Bozzalla Cassione, G. Zanframundo, A. Biglia, V. Codullo, C. Montecucco, and L. Cavagna, COVID-19 infection in a northern-Italian cohort of systemic lupus erythematosus assessed by telemedicine May 2020, Annals of the Rheumatic Diseases, Volume 79, Issue 10, Page 1382-1383
165 patient HCQ prophylaxis study: 50% more cases (p=0.59).
Survey of 165 SLE patients, 127 on HCQ. 8 patients with suspected COVID-19 and 4 confirmed cases. No mortality, one ICU case. 7 patients had no symptoms despite contact with a COVID-19 patient. No adjustment for concomitant medications or severity of SLE. Confounding by indication. https://c19p.org/cassione
342. J. Geleris, Y. Sun, J. Platt, J. Zucker, M. Baldwin, G. Hripcsak, A. Labella, D. Manson, C. Kubin, R. Barr, M. Sobieszczyk, and N. Schluger, Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19 May 2020, NEJM, May 7, 2020, Volume 382, Issue 25, Page 2411-2418
LATE TREATMENT 1,446 patient HCQ late treatment study: 4% higher combined mortality/intubation (p=0.76).
Before propensity matching, 38 control patients had hypertension. After propensity matching, 146 patients had hypertension (Table 1). Even if all propensity matched control patients had hypertension, the control prevalence would only be 14% compared to 49% for treatment. Since patients with hypertension are at much greater risk of mortality (HR 2.12), this appears to invalidate the results. Observational study of 1,446 hospitalized patients showing no significant effect on a combined intubation/death outcome for late treatment. However, a secondary analysis shows the success of HCQ was hidden by combining intubation and death – death / (combined death/intubation) for HCQ was 60% vs. control 89%. RCT recommended. No AZ or Zinc. HCQ group much sicker – patients already in mild/moderate acute respiratory distress, most of the control group not in acute respiratory distress. Control cases received other therapeutics. https://c19p.org/geleris
343. J. De la Iglesia, N. Fernández, R. Flores, M. Gómez, F. González de Haro, M. González, E. Vicente, M. Gil de Gómez, M. Guisado, I. Gómez, A. Andrada, N. Cao, P. Figaredo, C. García, L. Machón, Á. Alcalde, and J. Rilo, Hydroxicloroquine for pre-exposure prophyylaxis for SARS-CoV-2 Sep 2020, medRxiv
1,375 patient HCQ prophylaxis study: 43% more cases (p=0.15).
Analysis of autoimmune disease patients on HCQ, compared to a control group from the general population (matched on age and sex, but not adjusted for autoimmune disease), showing non-significant differences between groups. Other research shows that the risk of COVID-19 for systemic autoimmune disease patients is much higher overall, Ferri et al. show OR 4.42, p<0.001, which is the observed real-world risk, taking into account factors such as these patients potentially being more careful to avoid exposure. If adjusted for the different baseline risk, the mortality result becomes RR 0.35, p=0.23, suggesting a substantial benefit for HCQ treatment (as shown in other studies). https://c19p.org/delaiglesia
344. O. Uyaroğlu, M. Sönmezer, G. Telli Dizman, N. Çalık Başaran, S. Karahan, and Ö. Uzun, Comparison of Favipiravir to Hydroxychloroquine Plus Azithromycin in the Treatment of Patients with Non-critical COVID-19: A Single-center, Retrospective, Propensity Score-matched Study Mar 2022, Acta Medica, Volume 53, Issue 1, Page 73-82
LATE TREATMENT 84 patient HCQ late treatment PSM study: 200% higher mortality (p=1), 67% lower ICU admission (p=1), and 10% shorter hospitalization (p=0.9).
PSM retrospective 260 late stage hospitalized COVID-19 pneumonia patients in Turkey, showing no significant difference between favipiravir and HCQ. https://c19p.org/uyaroglu
345. A. Erden, O. Karakas, B. Armagan, S. Guven, B. Ozdemir, E. Atalar, H. Apaydin, E. Usul, I. Ates, A. Omma, and O. Kucuksahin, COVID-19 outcomes in patients with antiphospholipid syndrome: a retrospective cohort study Jan 2022, Bratislava Medical J., Volume 123, Issue 02, Page 120-124
9 patient HCQ prophylaxis study: 75% lower hospitalization (p=0.23).
Retrospective 9 COVID-19 patients with antiphospholipid syndrome in Turkey, showing no significant differences based on existing HCQ treatment. https://c19p.org/erden
346. P. Bhatt, V. Patel, P. Shah, and K. Parikh, Hydroxychloroquine Prophylaxis against Coronavirus Disease-19: Practice Outcomes among Health-Care Workers Aug 2021, medRxiv
927 patient HCQ prophylaxis study: 49% more cases (p=0.02).
Observational study of 927 low-risk healthcare workers in India, 731 volunteering for weekly HCQ prophylaxis, showing higher cases with treatment in unadjusted results. Clinical outcome was in the protocol, however no information on which patients were symptomatic is provided. There were no adverse events and no hospitalizations or deaths. Compliance was very low, decreasing weekly, with almost all participants discontinuing by week 11. The majority of infections occurred in later weeks when patient compliance was very low, and there was no per protocol analysis. #ECR/206/Inst/GJ/2013/RR-20. https://c19p.org/bhatt
347. H. Li, M. Deng, J. Wang, L. Ma, and Z. Yang, Treatment of COVID-19 patients with hydroxychloroquine or chloroquine: A retrospective analysis Jan 2021, Research Square
LATE TREATMENT 37 patient HCQ late treatment study: 40% slower viral clearance (p=0.06).
Small retrospective database analysis of 37 late stage patients hospitalized in an intensive care center in China, not finding a significant difference in viral shedding. Patients were all in serious condition. There was only one death however the group is not specified. Confounding by indication is likely. https://c19p.org/li2
348. A. Komissarov, I. Molodtsov, O. Ivanova, E. Maryukhnich, S. Kudryavtseva, A. Mazus, E. Nikonov, and E. Vasilieva, Hydroxychloroquine has no effect on SARS-CoV-2 load in nasopharynx of patients with mild form of COVID-19 Jun 2020, medRxiv
LATE TREATMENT 36 patient HCQ late treatment study: 25% worse viral load (p=0.45).
Small late stage (7-10 days post symptoms) study of nasal swab RNA with 12 control and 33 patients, showing no significant differences (significant reduction in viral load is seen in both groups). The groups are not comparable, with significant differences seen between hospitalized and non-hospitalized patients. 9 of 10 hospitalized patients were in the HCQ group and only one in the control group. 2 additional control patients were added between the first and second version of this preprint (including the only hospitalized control patient). https://c19p.org/komissarov
349. D. Guillaume, B. Magalie, E. Sina, S. Imène, V. Frédéric, D. Mathieu, M. Aurore, G. Yoni, E. Emma, B. Charlotte, F. Laura, S. Alain, N. Steven, Z. Pierre, F. Jean-Luc, C. Romain, G. Alice, M. Adrien, G. Wassim, R. Pierre-Emmanuel, P. Christophe, C. Catherine, B. Kevin, S. Thomas, and G. Damien, Antirheumatic Drug Intake Influence on Occurrence of COVID-19 Infection in Ambulatory Patients with Immune-Mediated Inflammatory Diseases: A Cohort Study Sep 2021, Rheumatology and Therapy, Volume 8, Issue 4, Page 1887-1895
459 patient HCQ prophylaxis study: 2% higher hospitalization (p=1) and 3% more cases (p=0.96).
Retrospective 459 lupus, rheumatoid, SjS, or psoriatic arthritis patients in France, showing no significant difference with HCQ treatment. However, the statistical analysis shows significant mismatches with prior research, which may be due to overfitting with the limited data and very small number of events. For example, the analysis estimates lower risk OR 0.72 for age, and shows very different relative risks of lupus, rheumatic, SjS, or psoriatic arthritis compared to other research. We note the very different distribution of diseases in the groups, for example there is a much higher prevalence of psoriatic arthritis in the no HCQ group. The inaccurate estimations of risk for the different diseases and for age likely makes the adjusted analysis very inaccurate. https://c19p.org/guillaume
350. M. Stewart, C. Rodriguez-Watson, A. Albayrak, J. Asubonteng, A. Belli, T. Brown, K. Cho, R. Das, E. Eldridge, N. Gatto, A. Gelman, H. Gerlovin, S. Goldberg, E. Hansen, J. Hirsch, Y. Ho, A. Ip, M. Izano, J. Jones, A. Justice, R. Klesh, S. Kuranz, C. Lam, Q. Mao, S. Mataraso, R. Mera, D. Posner, J. Rassen, A. Siefkas, A. Schrag, G. Tourassi, A. Weckstein, F. Wolf, A. Bhat, S. Winckler, E. Sigal, and J. Allen, COVID-19 Evidence Accelerator: A parallel analysis to describe the use of Hydroxychloroquine with or without Azithromycin among hospitalized COVID-19 patients Mar 2021, PLoS ONE, Volume 16, Issue 3, Page e0248128
LATE TREATMENT 11,157 patient HCQ late treatment study: 28% higher mortality (p=0.03) and 29% higher ventilation (p=0.09).
Retrospective analysis of seven databases in the USA, showing higher mortality with treatment. Results contradict strong evidence from the RECOVERY/SOLIDARITY trials, suggesting substantial confounding by indication. Time based confounding is very likely because HCQ became highly controversial and usage dramatically declined over the time covered, while overall treatment protocols during this period improved dramatically, i.e., more control patients likely come later in the period when treatment protocols were greatly improved. This study includes anyone PCR+ during or prior to their visit, and anyone with ICD-10 COVID-19 codes which includes asymptomatic PCR+ patients, therefore some patients in the control groups may be asymptomatic with regards to SARS-CoV-2, but in the hospital for another reason. Authors do not mention the possibility of any of these likely confounding factors. https://c19p.org/stewart
351. R. Vivanco-Hidalgo, I. Molina, E. Martinez, R. Roman-Viñas, A. Sánchez-Montalvá, J. Fibla, C. Pontes, and C. Velasco Muñoz, Incidence of COVID-19 in patients exposed to chloroquine and hydroxychloroquine: results from a population-based prospective cohort in Catalonia, Spain, 2020 Mar 2021, Eurosurveillance, Volume 26, Issue 9
20,238 patient HCQ prophylaxis study: 46% higher hospitalization (p=0.1) and 8% more cases (p=0.5).
Retrospective database analysis of chronic HCQ users and matched control patients, failing to match or adjust for the very different baseline risk for systemic autoimmune disease patients. Other research shows that the risk of COVID-19 for systemic autoimmune disease patients is much higher overall, Ferri et al. show OR 4.42, p<0.001. https://c19p.org/vivancohidalgo
352. M. Bosaeed, E. Mahmoud, A. Alharbi, H. Altayib, H. Albayat, F. Alharbi, K. Ghalilah, A. Al Arfaj, J. AlJishi, A. Alarfaj, H. Alqahtani, B. Almutairi, M. Almaghaslah, N. Alyahya, A. Bawazir, S. AlEisa, A. Alsaedy, A. Bouchama, M. Alharbi, M. AlShamrani, S. Al Johani, M. Aljeraisy, M. Alzahrani, A. Althaqafi, H. Almarhabi, A. Alotaibi, N. Alqahtani, Y. Arabi, O. Aldibasi, and A. Alaskar, Favipiravir and Hydroxychloroquine Combination Therapy in Patients with Moderate to Severe COVID19 (FACCT Trial): An Open-Label, Multicenter, Randomized, Controlled Trial Apr 2021, Infect. Dis. Ther., Volume 10, Issue 4, Page 2291-2307
LATE TREATMENT 254 patient HCQ late treatment RCT: 4% lower mortality (p=0.91), 8% higher ventilation (p=0.78), 31% higher ICU admission (p=0.24), and 29% slower recovery (p=0.29).
RCT 254 very late stage (93% on oxygen, 17% in ICU at baseline) hospitalized patients in Saudi Arabia not showing significant differences with HCQ+favipiravir treatment. Only SaO2 < 94% patients were eligible, however the actual SaO2 of enrolled patients is not provided. https://c19p.org/bosaeed
353. D. De Luna, Y. Roque, N. Batlle, K. Gómez, M. Jáquez, B. Cabrera, L. De la Cruz, O. Tavárez, R. Belliard, and J. Sanchez, Clinical and Demographic Characteristics of COVID-19 Patients Admitted in a Tertiary Care Hospital in the Dominican Republic Dec 2020, medRxiv
LATE TREATMENT 150 patient HCQ late treatment study: 105% higher mortality (p=0.69).
Retrospective 150 patients in the Dominican Republic, 132 treated with HCQ, showing higher mortality with treatment in unadjusted results. Confounding by indication is likely. https://c19p.org/deluna
354. D. Edwards and D. McGrail, COVID-19 Case Series at UnityPoint Health St. Luke’s Hospital in Cedar Rapids, IA Jul 2020, medRxiv
LATE TREATMENT 75 patient HCQ late treatment study: 70% higher mortality (p=0.69).
HCQ+AZ early in the epidemic had a fairly good success rate with few complications, 86% of HCQ patients survived and 92% of HCQ+AZ patients. Patients not receiving either had 93% survival but were not considered comparable because the treated groups were significantly more ill (100% hypoxic at admission vs. 59%) and this study does not adjust for the differences. Transition from an early intubation strategy to aggressive utilization of high flow nasal cannula and noninvasive ventilation (i.e, BiPAP) was successful in freeing up ICU resources. https://c19p.org/mcgrail
355. J. Barbosa, D. Kaitis, R. Freedman, K. Le, X. Lin, Clinical outcomes of hydroxychloroquine in hospitalized patients with COVID-19: a quasi-randomized comparative study Apr 2020, Preprint
LATE TREATMENT 63 patient HCQ late treatment study: 147% higher mortality (p=0.58).
Small retrospective study with 63 patients (32 treated with HCQ), showing no effectiveness, however the baseline state of each arm significantly differs. https://c19p.org/barbosa
356. S. Lotfy, A. Abbas, and W. Shouman, Use of Hydroxychloroquine in Patients with COVID-19: A Retrospective Observational Study Dec 2020, Turk. Thorac. J., Volume 22, Issue 1, Page 62-66
LATE TREATMENT 202 patient HCQ late treatment study: 25% higher mortality (p=0.76), 41% higher ventilation (p=0.34), and 17% higher ICU admission (p=0.53).
Retrospective 202 patients in Saudi Arabia not showing significant differences with treatment. No information is provided on how patients were selected for treatment, there may be significant confounding by indication. Time varying confounding is also likely as HCQ became controversial during the period studied, therefore HCQ use was likely more frequent toward the beginning of the period, a time when overall treatment protocols were significantly worse. https://c19p.org/lotfy
357. E. Burhan, K. Liu, E. Marwali, S. Huth, N. Wulung, D. Juzar, M. Taufik, S. Wijaya, D. Wati, N. Kusumastuti, S. Yuliarto, B. Pratomo, E. Pradian, D. Somasetia, D. Rusmawatiningtyas, A. Fatoni, J. Mandei, E. Lantang, F. Perdhana, B. Semedi, M. Rayhan, T. Tarigan, N. White, G. Bassi, J. Suen, and J. Fraser, Characteristics and outcomes of patients with severe COVID-19 in Indonesia: Lessons from the first wave Sep 2023, PLOS ONE, Volume 18, Issue 9, Page e0290964
LATE TREATMENT 559 patient HCQ ICU study: 1% higher mortality (p=0.91).
Retrospective 559 COVID-19 ICU patients in Indonesia, showing no difference in mortality with HCQ in unadjusted results. https://c19p.org/burhan
358. B. Silva, W. Rodrigues, D. Abadia, D. Alves da Silva, L. Andrade e Silva, C. Desidério, T. Farnesi-de-Assunção, J. Costa-Madeira, R. Barbosa, A. Bernardes e Borges, A. Hortolani Cunha, L. Pereira, F. Helmo, M. Lemes, L. Barbosa, R. Trevisan, M. Obata, G. Bueno, F. Mundim, A. Oliveira-Scussel, I. Monteiro, Y. Ferreira, G. Machado, K. Ferreira-Paim, H. Moraes-Souza, M. Da Silva, V. Rodrigues Júnior, and C. Oliveira, Clinical-Epidemiology Aspect of Inpatients With Moderate or Severe COVID-19 in a Brazilian Macroregion: Disease and Countermeasures May 2022, Frontiers in Cellular and Infection Microbiology, Volume 12
LATE TREATMENT 395 patient HCQ late treatment study: 46% higher mortality (p=0.22).
Retrospective 395 hospitalized patients in Brazil, showing higher mortality with HCQ treatment, without statistical significance. https://c19p.org/silva3
359. N. Kokturk, C. Babayigit, S. Kul, P. Duru Cetinkaya, S. Atis Nayci, S. Argun Baris, O. Karcioglu, P. Aysert, I. Irmak, A. Akbas Yuksel, Y. Sekibag, O. Baydar Toprak, E. Azak, S. Mulamahmutoglu, C. Cuhadaroglu, A. Demirel, B. Kerget, B. Baran Ketencioglu, H. Ozger, G. Ozkan, Z. Ture, B. Ergan, V. Avkan Oguz, O. Kilinc, M. Ercelik, T. Ulukavak Ciftci, O. Alici, E. Nurlu Temel, O. Ataoglu, A. Aydin, D. Cetiner Bahcetepe, Y. Gullu, F. Fakili, F. Deveci, N. Kose, M. Tor, G. Gunluoglu, S. Altin, T. Turgut, T. Tuna, O. Ozturk, O. Dikensoy, P. Yildiz Gulhan, I. Basyigit, H. Boyaci, I. Oguzulgen, S. Borekci, B. Gemicioglu, F. Bayraktar, O. Elbek et al., The predictors of COVID-19 mortality in a nationwide cohort of Turkish patients Apr 2021, Respiratory Medicine, Volume 183, Page 106433
LATE TREATMENT 1,500 patient HCQ late treatment study: 4% higher mortality (p=0.97).
Retrospective 1,500 hospitalized late stage (median SaO2 87.7) patients in Turkey, showing no significant difference with HCQ treatment. https://c19p.org/kokturk
360. D. Rivera, S. Peters, O. Panagiotou, D. Shah, N. Kuderer, C. Hsu, S. Rubinstein, B. Lee, T. Choueiri, G. De Lima Lopes, P. Grivas, C. Painter, B. Rini, M. Thompson, J. Arcobello, Z. Bakouny, D. Doroshow, P. Egan, D. Farmakiotis, L. Fecher, C. Friese, M. Galsky, S. Goel, S. Gupta, T. Halfdanarson, B. Halmos, J. Hawley, A. Khaki, C. Lemmon, S. Mishra, A. Olszewski, N. Pennell, M. Puc, S. Revankar, L. Schapira, A. Schmidt, G. Schwartz, S. Shah, J. Wu, Z. Xie, A. Yeh, H. Zhu, Y. Shyr, G. Lyman, and J. Warner, Utilization of COVID-19 Treatments and Clinical Outcomes among Patients with Cancer: A COVID-19 and Cancer Consortium (CCC19) Cohort Study Jul 2020, Cancer Discovery, Volume 10, Issue 10, Page 1514-1527
LATE TREATMENT 506 patient HCQ late treatment study: 2% higher mortality (p=0.92).
Retrospective cancer patients, showing adjusted OR 1.03 [0.62-1.73] for HCQ. The study reports the number of HCQ+AZ patients but they do not provide results for HCQ+AZ (only HCQ + any other treatment). Significant confounding by indication and compassionate use is likely. https://c19p.org/rivera
361. C. Chen, Y. Lin, T. Chen, T. Tseng, H. Wong, C. Kuo, W. Lin, S. Huang, W. Wang, J. Liao, C. Liao, Y. Hung, T. Lin, T. Chang, C. Hsiao, Y. Huang, W. Chung, C. Cheng, and S. Cheng, A Multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate Coronavirus disease 2019 (COVID-19) Jul 2020, PLoS ONE, Volume 15, Issue 12, Page e0242763
LATE TREATMENT 37 patient HCQ late treatment study: 29% worse viral clearance (p=0.7).
2 very small studies with hospitalized patients in Taiwan. RCT with 21 treatment and 12 standard-of-care patients. No mortality, or serious adverse effects. Median time to negative RNA 5 days versus 10 days standard-of-care, p=0.4. Risk of PCR+ at day 14, RR 0.76, p = 0.71. Small retrospective study with 12 of 28 HCQ patients and 5 of 9 in the control group being PCR- at day 14, RR 1.29, p = 0.7. The RCT and retrospective study are listed separately [Chen, Chen]. https://c19p.org/chen26
362. M. Mahévas, V. Tran, M. Roumier, A. Chabrol, R. Paule, C. Guillaud, E. Fois, R. Lepeule, T. Szwebel, F. Lescure, F. Schlemmer, M. Matignon, M. Khellaf, E. Crickx, B. Terrier, C. Morbieu, P. Legendre, J. Dang, Y. Schoindre, J. Pawlotsky, M. Michel, E. Perrodeau, N. Carlier, N. Roche, V. De Lastours, C. Ourghanlian, S. Kerneis, P. Ménager, L. Mouthon, E. Audureau, P. Ravaud, B. Godeau, S. Gallien, and N. Costedoat-Chalumeau, Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data May 2020, BMJ 2020, Page m1844
LATE TREATMENT 173 patient HCQ late treatment study: 20% higher mortality (p=0.75).
Observational study of 181 patients with advanced disease requiring oxygen showing no benefit for HCQ. Power of study appears too low to support conclusions, per the BMJ. None of the 15 patients receiving HCQ+AZ were transferred to intensive care or died compared to 23% overall. https://c19p.org/mahevas
363. E. Rosenberg, E. Dufort, T. Udo, L. Wilberschied, J. Kumar, J. Tesoriero, P. Weinberg, J. Kirkwood, A. Muse, J. DeHovitz, D. Blog, B. Hutton, D. Holtgrave, and H. Zucker, Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State May 2020, JAMA, May 11, 2020, Volume 323, Issue 24, Page 2493
LATE TREATMENT 1,483 patient HCQ late treatment study: 35% higher mortality (p=0.31).
Retrospective observational late stage study in New York showing no significant differences but calling for clinical trials. Zervos et al. point out serious limitations that they say should be corrected on the record: patients receiving HCQ with or without AZ were overall sicker on presentation and had multiple other risk factors including much higher risk based on ethnicity; patients receiving HCQ were more likely to be obese, diabetic, have chronic lung disease, and cardiovascular conditions; yet these sicker patients had approximately the same mortality rates compared to patients with a milder course of the disease and less risk factors. However, the authors conclude that “there are no significant benefits.” It is noteworthy that HCQ was associated with a significant survival benefit in a larger cohort of patients from New York City as reported by Mikami. Also see analysis piece at worldtribune.com. https://c19p.org/rosenberg
364. S. Auld, M. Caridi-Scheible, J. Blum, C. Robichaux, C. Kraft, J. Jacob, C. Jabaley, D. Carpenter, R. Kaplow, A. Hernandez-Romieu, M. Adelman, G. Martin, C. Coopersmith, and D. Murphy, ICU and ventilator mortality among critically ill adults with COVID-19 Apr 2020, Critical Care Medicine, Volume 48, Issue 9, Page e799-e804
LATE TREATMENT 217 patient HCQ late treatment study: 3% higher mortality (p=1).
Retrospective 217 critically ill patients, 114 receiving HCQ, showing no significant difference in mortality. https://c19p.org/auld
365. M. Souza-Silva, D. Pereira, M. Pires, I. Vasconcelos, A. Schwarzbold, D. Vasconcelos, E. Pereira, E. Manenti, F. Costa, F. Aguiar, F. Anschau, F. Bartolazzi, G. Nascimento, H. Vianna, J. Batista, J. Machado-Rugolo, K. Ruschel, M. Ferreira, L. Oliveira, L. Menezes, P. Ziegelmann, M. Tofani, M. Bicalho, M. Nogueira, M. Guimarães-Júnior, R. Aguiar, D. Rios, C. Polanczyk, and M. Marcolino, Dados de Vida Real sobre o Uso da Hidroxicloroquina ou da Cloroquina Combinadas ou Não à Azitromicina em Pacientes com Covid-19: Uma Análise Retrospectiva no Brasil Sep 2023, Arquivos Brasileiros de Cardiologia, Volume 120, Issue 9
LATE TREATMENT 1,346 patient HCQ late treatment study: 5% higher mortality (p=0.68), 21% higher ventilation (p=0.08), 9% higher ICU admission (p=0.31), and 12% longer hospitalization (p=0.03).
Retrospective 7,580 hospitalized patients in Brazil, showing longer hospitalization, and no significant difference in mortality, mechanical ventilation, and ICU admission with HCQ treatment. Authors note confounding by indication due to selected use in a compassionate use context. Authors match only on age, sex, cardiovascular comorbidities, and in-hospital use of corticosteroid, and only 10% of patients received HCQ/CQ, therefore confounding by indication is likely to be significant. A different matching list is included in the text, but neither includes COVID-19 severity. In the first line of the abstract authors falsely state that there is no evidence of benefit for HCQ treatment. While misrepresenting prior research is common, this is an extreme case and raises concern for validity of the analysis. In reality, controlled studies show statistically significant positive results for one or more outcomes (including RCTs). Authors discussion of prior research shows similar bias. https://c19p.org/souzasilva
366. K. Huh, W. Ji, M. Kang, J. Hong, G. Bae, R. Lee, Y. Na, and J. Jung, Association of prescribed medications with the risk of COVID-19 infection and severity among adults in South Korea Dec 2020, Int. J. Infectious Diseases, Volume 104, Page 7-14
44,046 patient HCQ prophylaxis study: 251% higher progression (p=0.11) and 6% fewer cases (p=0.82).
Retrospective database analysis with 17 cases for existing HCQ users and 5 severe cases, showing no significant difference for cases and higher risk for severe cases. However, HCQ users are likely systemic autoimmune disease patients and authors do not adjust for the very different baseline risk for these patients. Other research shows that the risk of COVID-19 for systemic autoimmune disease patients is much higher overall, Ferri et al. show OR 4.42, p<0.001. https://c19p.org/huh2
367. W. Ho, X. Wei, K. Tan, Y. Woh, M. Gill, A. Lok, S. Zulkifli, S. Idris, K. Khalid, L. Chee, and K. How, Hydroxychloroquine for COVID-19: A Single Center, Retrospective Cohort Study Mar 2023, Malaysian J. Medicine and Health Sciences, Volume 19, Issue 2, Page 8-13
LATE TREATMENT 325 patient HCQ late treatment study: 890% higher progression (p=0.03).
Retrospective 325 hospitalized COVID-19 patients in Malaysia, showing higher progression with HCQ, however the groups are not comparable. 17 HCQ vs. 3 control patients had severity category ≥3 at baseline (7 vs. 0 for severity ≥4). https://c19p.org/ho2
368. S. Civriz Bozdağ, G. Seval, İ. Yönal Hindilerden, F. Hindilerden, N. Andıç, M. Baydar, L. Aydın Kaynar, S. Toprak, H. Göksoy, B. Balık Aydın, U. Demirci, F. Can, V. Özkocaman, E. Gündüz, Z. Güven, Z. Özkurt, S. Demircioğlu, M. Beksaç, İ. İnce, U. Yılmaz, H. Eroğlu Küçükdiler, E. Abishov, B. Yavuz, Ü. Ataş, Y. Mutlu, V. Baş, F. Özkalemkaş, H. Üsküdar Teke, V. Gürsoy, S. Çelik, R. Çiftçiler, M. Yağcı, P. Topçuoğlu, Ö. Çeneli, H. Abbasov, C. Selim, M. Ar, O. Yücel, S. Sadri, C. Albayrak, A. Demir, N. Güler, M. Keklik, H. Terzi, A. Doğan, Z. Yegin, M. Kurt Yüksel, S. Sadri, İ. Yavaşoğlu, H. Beköz et al., Clinical Characteristics and Outcome of COVID-19 in Turkish Hematological Malignancy Patients Sep 2021, Turk. J. Haematol., Volume 39, Issue 1, Page 43-54
LATE TREATMENT 175 patient HCQ late treatment study: 399% higher mortality (p=0.003).
Retrospective 340 patients with hematological malignancy in Turkey, showing higher mortality with HCQ treatment. Confounding by time is likely because more HCQ patients were earlier in time when overall treatment protocols were significantly worse. https://c19p.org/civrizbozdag
369. M. Alotaibi, A. Ali, D. Bakhshwin, Y. Alatawi, S. Alotaibi, A. Alhifany, B. Alharthi, N. Alharthi, A. Alyazidi, Y. Alharthi, A. Alrafiah, Effectiveness and Safety of Favipiravir Compared to Hydroxychloroquine for Management of Covid-19: A Retrospective Study Sep 2021, Int. J. General Medicine
LATE TREATMENT 437 patient HCQ late treatment study: 134% higher mortality (p=0.05).
Retrospective hospitalized patients in Saudi Arabia, showing lower mortality with favipiravir compared to HCQ, not quite reaching statistical significance. Authors do not indicate the factors behind which therapy was chosen. May be subject to significant confounding by indication and confounding by time. https://c19p.org/alotaibi
370. R. Tamura, S. Said, L. De Freitas, and I. Rubio, Outcome and death risk of diabetes patients with Covid-19 receiving pre-hospital and in-hospital metformin therapies Jul 2021, Diabetology & Metabolic Syndrome, Volume 13, Issue 1
LATE TREATMENT 188 patient HCQ late treatment study: 299% higher mortality (p=0.04).
Retrospective 188 hospitalized patients in Brazil, showing higher risk of mortality with HCQ. Relatively few patients received HCQ. The results are likely subject to confounding by indication with treatment more likely for severe cases, and severity was not used in adjustments. Confounding by time is likely, with declining use of HCQ and improving standard-of-care over the study period. https://c19p.org/tamurah
371. A. Saib, W. Amara, P. Wang, S. Cattan, A. Dellal, K. Regaieg, S. Nahon, O. Nallet, and L. Nguyen, Lack of efficacy of hydroxychloroquine and azithromycin in patients hospitalized for COVID-19 pneumonia: A retrospective study Jun 2021, PLOS ONE, Volume 16, Issue 6, Page e0252388
LATE TREATMENT 104 patient HCQ late treatment PSM study: 125% higher combined mortality/intubation (p=0.23).
203 hospitalized patients in France, not showing significant differences with treatment. Confounding by indication is likely. Authors do not discuss confounding. https://c19p.org/saib
372. D. Sammartino, F. Jafri, B. Cook, L. La, H. Kim, J. Cardasis, and J. Raff, Predictors for inpatient mortality during the first wave of the SARS-CoV-2 pandemic: A retrospective analysis May 2021, PLOS One, Volume 16, Issue 5, Page e0251262
LATE TREATMENT 328 patient HCQ late treatment PSM study: 240% higher mortality (p=0.002).
Retrospective 1,108 hospitalized patients in New York showing significantly higher mortality with HCQ treatment. Time based confounding is very likely because HCQ became increasingly controversial and less used over the time covered (Mar – Jun 2020), while overall treatment protocols during this period improved dramatically, i.e., more control patients likely come later in the period when treatment protocols were greatly improved. Authors note that for every week or month later that a person was admitted, their risk of death dropped by 16% and 49%, respectively, yet they do not consider time based confounding. https://c19p.org/sammartino
373. P. Mohandas, S. Periasamy, M. Marappan, A. Sampath, V. Garfin Sundaram, and V. Cherian, Clinical review of COVID-19 patients presenting to a quaternary care private hospital in South India: A retrospective study Apr 2021, Clinical Epidemiology and Global Health, Volume 11, Page 100751
LATE TREATMENT 3,345 patient HCQ late treatment study: 81% higher mortality (p=0.007).
Retrospective 3,345 hospitalized patients in India, 11.5% treated with HCQ, showing unadjusted higher mortality with treatment. Confounding by indication and time based confounding (due to declining use over the period when overall treatment protocols improved dramatically) are likely. https://c19p.org/mohandas
374. K. Sands, R. Wenzel, L. McLean, K. Korwek, J. Roach, K. Miller, R. Poland, L. Burgess, E. Jackson, and J. Perlin, No clinical benefit in mortality associated with hydroxychloroquine treatment in patients with COVID-19 Dec 2020, Int. J. Infectious Diseases, Volume 104, Page 34-40
LATE TREATMENT 1,669 patient HCQ late treatment study: 70% higher mortality (p=0.01).
Retrospective database analysis of 1,669 patients in the US showing OR 1.81, p = 0.01. Confounding by indication is likely. COVID-19 was determined via PCR+ results, therefore authors include patients asymptomatic for COVID-19, but in the hospital for other reasons. While authors adjust for severity, the method used is very poor. 93.5% of patients are classified as “mild” which is patients with no documented care in a critical care unit within 8 hours of admission. Therefore, almost all patients are in the same category, and those in a different category may be due to symptoms unrelated to COVID-19. Lower bias toward male patients in the control group also agrees with the hypothesis that the control group is made up of more people that were in hospital for another reason. Since the analysis covers the initial period of the pandemic in the USA, it is likely that HCQ was used more often earlier in the analysis period when treatment protocols were considerably worse. A lengthy critique of the significant shortcomings (and obvious errors) are noted in a International Journal of Infectious Disease published letter to the editor. https://c19p.org/sands
375. G. Psevdos, A. Papamanoli, and Z. Lobo, Corona Virus Disease-19 (COVID-19) in a Veterans Affairs Hospital at Suffolk County, Long Island, New York Dec 2020, Open Forum Infectious Diseases, Volume 7, Issue Supplement_1, Page S330-S331
LATE TREATMENT 67 patient HCQ late treatment study: 63% higher mortality (p=0.52).
Retrospective 67 hospitalized patients in the USA showing non-statistically significant unadjusted increased mortality with HCQ. Confounding by indication is likely. Time varying confounding is likely. HCQ became controversial and was suspended during the end of the period studied, therefore HCQ use was likely more frequent toward the beginning of the study period, a time when overall treatment protocols were significantly worse. https://c19p.org/psevdos
376. C. Teixeira, H. Shiflett, D. Jandhyala, J. Lewis, S. Curry, and C. Salgado, Characteristics and outcomes of COVID-19 patients admitted to a regional health system in the southeast Dec 2020, Open Forum Infectious Diseases, Volume 7, Issue Supplement_1, Page S251-S253
LATE TREATMENT 161 patient HCQ late treatment study: 79% higher mortality (p=0.1).
Retrospective 161 hospitalized patients in the USA showing non-statistically significant unadjusted increased mortality with HCQ. Confounding by indication is likely. Time varying confounding is likely. HCQ became controversial and was suspended towards the end of the period studied, therefore HCQ use was likely more frequent toward the beginning of the study period, a time when overall treatment protocols were significantly worse. https://c19p.org/teixeira
377. SOLIDARITY Trial Consortium et al., Repurposed antiviral drugs for COVID-19; interim WHO SOLIDARITY trial results Oct 2020, SOLIDARITY Trial Consortium, NEJM, Volume 384, Issue 6, Page 497-511
LATE TREATMENT 1,853 patient HCQ late treatment RCT: 19% higher mortality (p=0.23).
WHO SOLIDARITY open-label trial with 954 very late stage (64% on oxygen/ventilation) HCQ patients, mortality relative risk RR 1.19 [0.89-1.59], p=0.23. HCQ dosage very high as in RECOVERY, 1.6g in the first 24 hours, 9.6g total over 10 days, only 25% less than the high dosage that Borba et al. show greatly increases risk (OR 2.8). Authors state they do not know the weight or obesity status of patients to analyze toxicity (since they do not adjust dosage based on patient weight, toxicity may be higher in patients of lower weight). KM curves show a spike in HCQ mortality days 5-7, corresponding to ~90% of the total excess seen at day 28 (a similar spike is seen in the RECOVERY trial). Almost all excess mortality is from ventilated patients. Authors refer to a lack of excess mortality in the first few days to suggest a lack of toxicity, but they are ignoring the very long half-life of HCQ and the dosing regimen – much higher levels of HCQ will be reached later. Increased mortality after 2 days of HCQ dosing in Borba et al. An unspecified percentage of patients used the more toxic CQ. No placebo used. Atypically high doses were used regardless of weight, so concentrations vary substantially in different tissues and lung concentration may be >30x plasma concentration. https://c19p.org/solidarity
378. M. Laplana, O. Yuguero, and J. Fibla, Lack of protective effect of chloroquine derivatives on COVID-19 disease in a Spanish sample of chronically treated patients Sep 2020, PLOS ONE, Volume 15, Issue 12, Page e0243598
638 patient HCQ prophylaxis study: 56% more cases (p=0.24).
Survey of 319 autoimmune disease patients taking CQ/HCQ with 5.3% COVID-19 incidence, compared to a control group from the general population (matched on age, sex, and region, but not adjusted for autoimmune disease), with 3.4% incidence. Its not clear why authors did not compare with autoimmune patients not on CQ/HCQ. Other research shows that the risk of COVID-19 for systemic autoimmune disease patients is much higher overall. Ferri et al. show OR 4.42, p<0.001, which is the observed real-world risk, taking into account factors such as these patients potentially being more careful to avoid exposure. If we adjust for the different baseline risk, the result becomes RR 0.36, p<0.001, suggesting a substantial benefit for HCQ/CQ treatment (as shown in other studies). There may also be significant survey bias – those experiencing COVID-19 may be more likely to respond to the survey. Authors note that they “could not eliminate completely the possibility of some bias due to the intrinsic condition of the individuals within the treatment group that are undergoing chloroquine or derivative drug treatment due to other diseases that alter their health status and may have different comorbidities” however they could account for one significant bias by comparing with matched autoimmune disease patients. https://c19p.org/laplana
379. M. Kelly, R. O’Connor, L. Townsend, M. Coghlan, E. Relihan, M. Moriarty, B. Carr, G. Melanophy, C. Doyle, C. Bannan, R. O’Riordan, C. Merry, S. Clarke, and C. Bergin, Clinical outcomes and adverse events in patients hospitalised with COVID-19, treated with off-label hydroxychloroquine and azithromycin Jul 2020, British J. Clinical Pharmacology, Volume 87, Issue 3, Page 1150-1154
LATE TREATMENT 134 patient HCQ late treatment study: 143% higher mortality (p=0.03).
Retrospective 82 hospitalized patients HCQ/AZ, 52 standard-of-care, not finding statistically significant differences. Confounding by indication – authors note that the HCQ/AZ patients were more severely ill, and do not attempt to adjust for confounders. https://c19p.org/kelly
380. P. Cravedi, S. Mothi, Y. Azzi, M. Haverly, S. Farouk, M. Pérez-Sáez, M. Redondo-Pachón, B. Murphy, S. Florman, L. Cyrino, M. Grafals, S. Venkataraman, X. Cheng, A. Wang, G. Zaza, A. Ranghino, L. Furian, J. Manrique, U. Maggiore, I. Gandolfini, N. Agrawal, H. Patel, E. Akalin, and L. Riella, COVID-19 and kidney transplantation: Results from the TANGO International Transplant Consortium Jul 2020, American J. Transplantation, Volume 20, Issue 11, Page 3140-3148
LATE TREATMENT 144 patient HCQ late treatment study: 53% higher mortality (p=0.17).
Analysis of 144 hospitalized kidney transplant patients showing HCQ mortality HR 1.53, p = 0.17. Subject to confounding by indication. https://c19p.org/cravedi
381. N. Kuderer, T. Choueiri, D. Shah, Y. Shyr, S. Rubinstein, D. Rivera, S. Shete, C. Hsu, A. Desai, G. De Lima Lopes, P. Grivas, C. Painter, S. Peters, M. Thompson, Z. Bakouny, G. Batist, T. Bekaii-Saab, M. Bilen, N. Bouganim, M. Larroya, D. Castellano, S. Del Prete, D. Doroshow, P. Egan, A. Elkrief, D. Farmakiotis, D. Flora, M. Galsky, M. Glover, E. Griffiths, A. Gulati, S. Gupta, N. Hafez, T. Halfdanarson, J. Hawley, E. Hsu, A. Kasi, A. Khaki, C. Lemmon, C. Lewis, B. Logan, T. Masters, R. McKay, R. Mesa, A. Morgans, M. Mulcahy, O. Panagiotou, P. Peddi, N. Pennell, K. Reynolds et al., Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study May 2020, Lancet, June 20, 2020, Volume 395, Issue 10241, Page 1907-1918
LATE TREATMENT 928 patient HCQ late treatment study: 134% higher mortality (p<0.0001).
Retrospective 928 cancer patients, showing HCQ OR 1.06 [0.51-2.20]. HCQ+AZ OR 2.93 [1.79-4.79]. The relative risks of different therapies suggest that the results are overly affected by confounding by indication. Authors note: HCQ+AZ might not be the cause of increased mortality, but instead these were given to patients with more severe COVID-19. https://c19p.org/kuderer
382. L. Trefond, E. Drumez, M. Andre, N. Costedoat-Chalumeau, R. Seror, M. Devaux, E. Dernis, Y. Dieudonne, S. El Mahou, A. Lanteri, I. Melki, V. Queyrel, M. Roumier, J. Schmidt, T. Barnetche, T. Thomas, P. Cacoub, A. Belot, O. Aumaitre, C. Richez, and E. Hachulla, Effet d’un traitement par hydroxychloroquine prescrit comme traitement de fond de rhumatismes inflammatoires chroniques ou maladies auto-immunes systémiques sur les tests diagnostiques et l’évolution de l’infection à SARS CoV-2: étude de 871 patients Jan 2021, Revue du Rhumatisme, Volume 89, Issue 2, Page 192-195
262 patient HCQ prophylaxis study: 17% higher mortality (p=0.8), 78% higher combined mortality/ICU admission (p=0.21), and 45% higher hospitalization (p=0.12).
Retrospective 71 chronic HCQ patients compared with 191 matched controls, analyzing only those with a highly suspected or confirmed diagnosis of COVID-19. No significant difference was found in outcomes, however matching failed with extreme confounding – 77.5% of HCQ patients with systemic autoimmune diseases vs. 21.5% of control patients. Other research shows that the risk of COVID-19 for systemic autoimmune disease patients is much higher overall, Ferri et al. show OR 4.42, p<0.001. https://c19p.org/trefond
383. RECOVERY Collaborative Group et al., Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial Jun 2020, RECOVERY Collaborative Group, NEJM, Volume 383, Issue 21, Page 2030-2040
LATE TREATMENT 4,716 patient HCQ late treatment RCT: 9% higher mortality (p=0.15) and 15% higher ventilation (p=0.19).
RECOVERY trial finds no significant benefit for very late stage (9 days after symptom onset) very sick patients. Results may be due to the unusually high dosage used (9.2g total over 10 days). The overall dosage used is only 23% less than the high dosage that Borba et al. used, which shows a great increase in risk (OR 2.8). Authors do not report results based on weight, BMI, or related conditions such as diabetes, which may provide additional evidence of toxic dosages. Authors do not adjust dosage based on patient weight, so toxicity may be higher in patients of lower weight. KM curves show a spike in HCQ mortality days 5-8, corresponding to ~85% of the total excess seen at day 28 (a similar spike is seen in the SOLIDARITY trial). Authors note: “we did not observe excess mortality in the first 2 days of treatment … when early effects of dose-dependent toxicity might be expected” but they are ignoring the very long half-life of HCQ and the dosing regimen – much higher levels of HCQ will be reached later. Increased mortality in Borba et al. occurred after 2 days. Patients were extremely sick (median 9 days post symptoms, 60% requiring oxygen and an additional 17% requiring ventilation/ extracorporeal membrane oxygenation (extremely high risk medical intervention), with an unusually high mortality rate was seen in both arms. 1,561 HCQ patients, 3,155 standard-of-care. A secondary analysis has found several inconsistencies in the data. Hypoxia may inhibit HCQ entering cells, making it less effective for late stage use. See here for more on excessive HCQ dosing, also noting that concentrations vary substantially in different tissues and lung concentration may be >30x plasma concentration. https://c19p.org/recovery
384. S. Juneja, P. Rana, P. Chawala, R. Katoch, K. Singh, S. Rana, T. Mittal, B. Kaur, and S. Kaur, Hydroxychloroquine pre-exposure prophylaxis provides no protection against COVID-19 among health care workers: a cross-sectional study in a tertiary care hospital in North India Jan 2022, J. Basic and Clinical Physiology and Pharmacology, Volume 0, Issue 0
2,200 patient HCQ prophylaxis study: 142% higher severe cases (p=0.59) and 6% more cases (p=0.67).
Retrospective 2,200 healthcare workers in India, 996 taking HCQ prophylaxis, showing no significant differences. There were large differences in the occupation of participants and therefore exposure, and the authors make no adjustments. https://c19p.org/juneja
385. N. Awad, D. Schiller, M. Fulman, and A. Chak, Impact of hydroxychloroquine on disease progression and ICU admissions in patients with SARS-CoV-2 infection Feb 2021, American J. Health-System Pharmacy, Volume 78, Issue 8, Page 689-696
LATE TREATMENT 336 patient HCQ late treatment study: 19% higher mortality (p=0.6), 461% higher ventilation (p<0.0001), and 463% higher ICU admission (p<0.0001).
This paper has inconsistent values – the number of treatment and control patients differs in the text and Table 1, we have used treatment 188 and control 148. Retrospective 336 hospitalized patients in the USA showing higher mortality, ICU admission, and intubation with treatment. Confounding by indication is likely. Time varying confounding is also likely due to declining usage over the early period when overall treatment protocols were also improving dramatically. Authors and reviewers appear to be unfamiliar with either of these. https://c19p.org/awad
386. M. Oztas, M. Bektas, I. Karacan, N. Aliyeva, A. Dag, S. Aghamuradov, S. Cevirgen, S. Sari, M. Bolayirli, G. Can, G. Hatemi, E. Seyahi, H. Ozdogan, A. Gul, and S. Ugurlu, Frequency and Severity of COVID-19 in Patients with Various Rheumatic Diseases Treated Regularly with Colchicine or Hydroxychloroquine Mar 2022, J. Medical Virology
650 patient HCQ prophylaxis study: 215% higher hospitalization (p=0.36), 40% more symptomatic cases (p=0.44), and 5% more cases (p=0.88).
Retrospective 317 HCQ users and 333 household contacts, showing higher risk with HCQ. https://c19p.org/oztas
387. H. Gerlovin, D. Posner, Y. Ho, C. Rentsch, J. Tate, J. King, K. Kurgansky, I. Danciu, L. Costa, F. Linares, I. Goethert, D. Jacobson, M. Freiberg, E. Begoli, S. Muralidhar, R. Ramoni, G. Tourassi, J. Gaziano, A. Justice, D. Gagnon, and K. Cho, Pharmacoepidemiology, Machine Learning and COVID-19: An intent-to-treat analysis of hydroxychloroquine, with or without azithromycin, and COVID-19 outcomes amongst hospitalized US Veterans Jun 2021, American J. Epidemiology, Volume 190, Issue 11, Page 2405-2419
LATE TREATMENT 1,199 patient HCQ late treatment study: 22% higher mortality (p=0.18) and 55% higher ventilation (p=0.02).
Retrospective 1,769 hospitalized patients in the USA showing no significant differences for HCQ, and higher intubation for HCQ+AZ. https://c19p.org/gerlovin
388. L. Shahrin, M. Mahfuz, M. Rahman, M. Hossain, A. Khandaker, M. Alam, D. Osmany, M. Islam, M. Chisti, C. Ahmed, and T. Ahmed, Hospital-Based Quasi-Experimental Study on Hydroxychloroquine Pre-Exposure Prophylaxis for COVID-19 in Healthcare Providers with Its Potential Side-Effects Dec 2022, Life, Volume 12, Issue 12, Page 2047
336 patient HCQ prophylaxis study: 88% more cases (p=0.09).
Retrospective 230 low risk healthcare workers taking HCQ prophylaxis, and 106 that declined, showing higher cases without statistical significance. No case severity information is provided. The point estimate favored HCQ when excluding the first 14 days and including participants that worked for at least 16 days. Authors note a significant dose response relationship. https://c19p.org/shahrin
389. H. Burdick, C. Lam, S. Mataraso, A. Siefkas, G. Braden, R. Dellinger, A. McCoy, J. Vincent, A. Green-Saxena, G. Barnes, J. Hoffman, J. Calvert, E. Pellegrini, and R. Das, Is Machine Learning a Better Way to IdentifyCOVID-19 Patients Who Might Benefit fromHydroxychloroquineTreatment?—The IDENTIFY Trial Nov 2020, J. Clinical Medicine, Volume 9, Issue 12, Page 3834
LATE TREATMENT 290 patient HCQ late treatment study: 59% higher mortality (p=0.12).
290 patient observational trial in the USA, not showing a significant difference with HCQ treatment overall, but showing significantly lower mortality in a subgroup of patients where HCQ is expected to be beneficial based on a machine learning algorithm. https://c19p.org/burdick
390. J. Pablos, M. Galindo, L. Carmona, A. Lledó, M. Retuerto, R. Blanco, M. Gonzalez-Gay, D. Martinez-Lopez, I. Castrejón, J. Alvaro-Gracia, D. Fernández Fernández, A. Mera-Varela, S. Manrique-Arija, N. Mena Vázquez, and A. Fernandez-Nebro, Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study Aug 2020, Annals of the Rheumatic Diseases, Volume 79, Issue 12, Page 1544-1549
LATE TREATMENT 228 patient HCQ late treatment study: 126% higher severe cases (p=0.002).
Retrospective 228 rheumatic disease and 228 non-rheumatic disease hospitalized COVID-19 patients in Spain, showing higher risk of severe COVID-19 with HCQ treatment. https://c19p.org/pablos
391. M. Kalligeros, F. Shehadeh, E. Atalla, E. Mylona, S. Aung, A. Pandita, J. Larkin, M. Sanchez, F. Touzard-Romo, A. Brotherton, R. Shah, C. Cunha, and E. Mylonakis, Hydroxychloroquine use in hospitalised patients with COVID-19: An observational matched cohort study Aug 2020, J. Global Antimicrobial Resistance, Volume 22, Page 842-844
LATE TREATMENT 108 patient HCQ late treatment study: 67% higher mortality (p=0.57).
Small retrospective database analysis of 36 patients receiving HCQ not showing significant differences. Confounding by indication is likely. https://c19p.org/kalligeros
392. J. Mallat, F. Hamed, M. Balkis, M. Mohamed, M. Mooty, A. Malik, A. Nusair, and M. Bonilla, Hydroxychloroquine is associated with slower viral clearance in clinical COVID-19 patients with mild to moderate disease: A retrospective study May 2020, Medicine, Volume 99, Issue 52, Page e23720
LATE TREATMENT 34 patient HCQ late treatment study: 203% slower viral clearance (p=0.02).
Very small retrospective analysis of 34 patients finding slower binary PCR viral clearance with HCQ. No information on severity for treatment versus control is provided. No deaths, ICU admission, or mechanical ventilation. Binary PCR does not distinguish replication-competence. HCQ treatment started very late for many patients with >= 9 days for 25%. https://c19p.org/mallat
393. B. Tirupakuzhi Vijayaraghavan, V. Jha, D. Rajbhandari, S. Myatra, A. Ghosh, A. Bhattacharya, S. Arfin, A. Bassi, L. Donaldson, N. Hammond, O. John, R. Joshi, M. Kunigari, C. Amrutha, S. Husaini, S. Ghosh, S. Nag, H. Selvaraj, V. Kantroo, K. Shah, and B. Venkatesh, Hydroxychloroquine plus personal protective equipment versus personal protective equipment alone for the prevention of laboratory-confirmed COVID-19 infections among healthcare workers: a multicentre, parallel-group randomised controlled trial from India May 2022, BMJ Open, Volume 12, Issue 6, Page e059540
414 patient HCQ prophylaxis RCT: 196% higher progression (p=1), 52% lower hospitalization (p=0.62), and 14% fewer cases (p=0.73).
Low-dose prophylaxis RCT with low-risk healthcare workers in India, showing no significant differences. Symptomatic case results are not provided. Follow up was over 6 months, however treatment ended after 3 months. 21% of patients discontinued treatment before 3 months (Table S2). https://c19p.org/tirupakuzhi
394. R. Ferreira, R. Beranger, P. Sampaio, J. Mansur Filho, and R. Lima, Outcomes associated with Hydroxychloroquine and Ivermectin in hospitalized patients with COVID-19: a single-center experience Nov 2021, Revista da Associação Médica Brasileira, Volume 67, Issue 10, Page 1466-1471
LATE TREATMENT 192 patient HCQ late treatment study: 151% higher mortality (p=0.03) and 46% higher combined mortality/intubation (p=0.23).
Retrospective 230 hospitalized patients in Brazil showing higher mortality with HCQ treatment. Authors note that the treatments were more likely to be offered to sicker patients. Authors note that they do not know if treatment was started before or after ICU admission and intubation. Dosage is unknown. https://c19p.org/ferreira2h
395. A. Spivak, B. Barney, T. Greene, R. Holubkov, C. Olsen, J. Bridges, R. Srivastava, B. Webb, F. Sebahar, A. Huffman, C. Pacchia, J. Dean, and R. Hess, A Randomized Clinical Trial Testing Hydroxychloroquine for Reduction of SARS-CoV-2 Viral Shedding and Hospitalization in Early Outpatient COVID-19 Infection Mar 2023, Microbiology Spectrum, Volume 11, Issue 2
LATE TREATMENT 367 patient HCQ late treatment RCT: 73% higher hospitalization (p=0.54), 20% improved recovery (p=0.19), and 17% improved viral clearance (p=0.19).
Delayed publication of an early terminated late treatment RCT with low-risk (no mortality) outpatients in the USA, showing no significant differences with HCQ. Authors do not provide symptom onset data, but the subgroup analysis suggests that more patients may have been in the 5+ days group (the estimate for the 5+ days group has a smaller confidence interval, and the overall mean/median for HCQ is much closer to the 5+ days group). Treatment was started one day after enrollment according to Table S1 (authors report “commonly 1 day after randomization” in the text). This suggests that most patients were treated 6+ days after onset. Subgroup analysis for <5, ≥5 days is provided only for viral shedding duration, and shows improved results for earlier treatment. Adherence was only 66% (Figure 1). Publication was 21 months after the trial ended. Registered outcomes were modified November 2022, December 2022, and January 2023, all over a year after completion of the trial. For example, in January 2023, the household acquisition outcome at 28 days was deleted, leaving only 14 days. There are 7 versions of the statistical analysis plan, all dated after the start of the trial, and 5 dated after the completion of the trial. Many outcomes in the SAP are missing, including 6 month mortality and hospitalization, QOL, and KM for hospitalization/mortality. Notably, authors provide the age subgroup analysis for symptom scores and transmission, but they do not provide the time from onset analysis. Lack of symptom onset details, lack of onset subgroup analysis for clinical outcomes, and authors’ incorrect claim that none of the RCTs to date show “meaningful clinical outcomes” suggests significant bias. https://c19p.org/spivak
396. A. Schmidt, M. Tucker, Z. Bakouny, C. Labaki, C. Hsu, Y. Shyr, A. Armstrong, T. Beer, R. Bijjula, M. Bilen, C. Connell, S. Dawsey, B. Faller, X. Gao, B. Gartrell, D. Gill, S. Gulati, S. Halabi, C. Hwang, M. Joshi, A. Khaki, H. Menon, M. Morris, M. Puc, K. Russell, D. Shah, N. Shah, N. Sharifi, J. Shaya, M. Schweizer, J. Steinharter, E. Wulff-Burchfield, W. Xu, J. Zhu, S. Mishra, P. Grivas, B. Rini, J. Warner, T. Zhang, T. Choueiri, S. Gupta, R. McKay, A. Desai, A. Cohen, A. Olszewski, A. Bardia, A. Daher, A. Brown, A. Yeh, A. Hsiao et al., Association Between Androgen Deprivation Therapy and Mortality Among Patients With Prostate Cancer and COVID-19 Nov 2021, JAMA Network Open, Volume 4, Issue 11, Page e2134330
LATE TREATMENT 477 patient HCQ late treatment PSM study: 333% higher mortality (p=0.0001) and 613% higher severe cases (p<0.0001).
Retrospective 1,106 prostate cancer patients, showing higher mortality with HCQ treatment. https://c19p.org/schmidth
397. R. Barnabas, E. Brown, A. Bershteyn, H. Stankiewicz Karita, C. Johnston, L. Thorpe, A. Kottkamp, K. Neuzil, M. Laufer, M. Deming, M. Paasche-Orlow, P. Kissinger, A. Luk, K. Paolino, R. Landovitz, R. Hoffman, T. Schaafsma, M. Krows, K. Thomas, S. Morrison, H. Haugen, L. Kidoguchi, M. Wener, A. Greninger, M. Huang, K. Jerome, A. Wald, C. Celum, H. Chu, and J. Baeten, Hydroxychloroquine for Post-exposure Prophylaxis to Prevent Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Trial Dec 2020, Annals of Internal Medicine, Volume 174, Issue 3, Page 344-352
829 patient HCQ prophylaxis RCT: 27% more cases (p=0.33).
Early terminated pre-exposure prophylaxis RCT comparing HCQ and vitamin C with 781 low-risk patients (83% household contacts), reporting no significant differences. Different results were reported at IDWeek conference vs. above Annals of Internal Medicine publication. The study enrolled people with their last exposure within 4 days, i.e., if someone was exposed for 30 days in a row, they could be enrolled anywhere from day 1 to day 34. Therefore, many were likely infected earlier than the enrollment date. Note that PCR has a very high false negative rates, e.g., 100% on day 1 and 67% on day 4. 50% of infections were detected by day 4. With the PCR false negatives and treatment delays it is likely that most infections happened before enrollment or before HCQ can reach therapeutic levels. Significantly more cases were caught at baseline in the control group (54 vs. 29 for HCQ) and excluded from analysis. The early presentation stated that therapy started one day after enrollment and study supplies were sent to the participant “either by courier or mail.” The published paper changes this to “courier delivery within 48 hours.” Overall delays are unclear but may be: time since first exposure – unlimited time from last exposure to enrollment – 10% reported as >= 5 days’ time to telehealth meeting – 1 day (3 days if Friday enrollment?) time to receive medication – <48 hours (including weekends?) Symptomatic in this study was based on CDC-defined symptoms which contain symptoms that may be due to HCQ side effects. Some results have not been reported, including symptomatic @28 days. The study uses a low dosage over an extended period, therapeutic levels may only be reached nearer to day 14, if at all, so day 28 results should be more informative when available (although labeled a PEP trial, with the low dosage and continuous exposure for most participants it is more of a PrEP/PEP trial where benefit might be seen later as HCQ levels increase). Endpoints were: Primary outcomes: PCR+ @28 days mITT – aHR 1.16 [0.77-1.73] PCR+ @14 days mITT – aHR 1.10 [0.73-1.66] IDWeek report was different: aHR 0.99 [0.64-1.52] PCR+ @14 days ITT – aHR 0.81 [0.57-1.14] Secondary outcomes: PCR+ symptomatic @28 days – NOT REPORTED YET duration of shedding – NOT REPORTED YET. Not in study protocol: PCR+ cumulative symptomatic @14 days – aHR 1.23 [0.76-1.99]. Dose in first 24 hours – 0.8g (compare with Boulware et al. 2g) Dose in first 5 days – 1.6g (compare with Boulware et al. 3.8g) Other research suggests vitamin C may be beneficial for COVID-19. No information on severity of cases is provided. Binary PCR does not distinguish replication-competence. There were 2 COVID-19 hospitalizations, one in each group. Side effects were similar for HCQ and placebo. 83% medication adherence at day 14. Primary Funding Source Bill & Melinda Gates Foundation. COVID-19 PEP. NCT04328961. https://c19p.org/barnabas
398. W. Self, M. Semler, L. Leither, J. Casey, D. Angus, R. Brower, S. Chang, S. Collins, J. Eppensteiner, M. Filbin, D. Files, K. Gibbs, A. Ginde, M. Gong, F. Harrell, D. Hayden, C. Hough, N. Johnson, A. Khan, C. Lindsell, M. Matthay, M. Moss, P. Park, T. Rice, B. Robinson, D. Schoenfeld, N. Shapiro, J. Steingrub, C. Ulysse, A. Weissman, D. Yealy, B. Thompson, and S. Brown, Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19: A Randomized Clinical Trial Nov 2020, JAMA, Volume 324, Issue 21, Page 2165
LATE TREATMENT 477 patient HCQ late treatment RCT: 6% higher mortality (p=0.85) and 3% worse 7-point scale results (p=0.87).
Early terminated very late stage (65% on supplemental oxygen) RCT with 242 HCQ and 237 control patients showing no significant difference in outcomes. For the subgroup not on supplemental oxygen at baseline (relatively early treatment), the odds ratio for the 7-point outcome scale is: adjusted odds ratio 0.61 [0.34-1.08]. https://c19p.org/self
399. R. Ulrich, A. Troxel, E. Carmody, J. Eapen, M. Bäcker, J. DeHovitz, P. Prasad, Y. Li, C. Delgado, M. Jrada, G. Robbins, B. Henderson, A. Hrycko, D. Delpachitra, V. Raabe, J. Austrian, Y. Dubrovskaya, and M. Mulligan, Treating Covid-19 With Hydroxychloroquine (TEACH): A Multicenter, Double-Blind, Randomized Controlled Trial in Hospitalized Patients Sep 2020, Open Forum Infectious Diseases, Volume 7, Issue 10
LATE TREATMENT 128 patient HCQ late treatment RCT: 6% higher mortality (p=1) and 173% higher ICU admission (p=0.13).
Small RCT on very late stage use of HCQ, with 48% on oxygen at baseline. 67 HCQ patients, 61 control. Baseline states were not comparable – 82% more HCQ patients had the highest severity at baseline, there was 32% more male HCQ patients, and 44% more control patients used AZ. The HCQ group also had significantly more patients with cerebrovascular disease, cardiovascular disease (non-hypertension), renal disease (non-dialysis), and a history of organ transplants. https://c19p.org/ulrich
400. C. Babayigit, N. Kokturk, S. Kul, P. Cetinkaya, S. Atis Nayci, S. Argun Baris, O. Karcioglu, P. Aysert, I. Irmak, A. Akbas Yuksel, Y. Sekibag, O. Baydar Toprak, E. Azak, S. Mulamahmutoglu, C. Cuhadaroglu, A. Demirel, B. Kerget, B. Baran Ketencioglu, H. Ozger, G. Ozkan, Z. Ture, B. Ergan, V. Avkan Oguz, O. Kilinc, M. Ercelik, T. Ulukavak Ciftci, O. Alici, E. Nurlu Temel, O. Ataoglu, A. Aydin, D. Cetiner Bahcetepe, Y. Gullu, F. Fakili, F. Deveci, N. Kose, M. Tor, G. Gunluoglu, S. Altin, T. Turgut, T. Tuna, O. Ozturk, O. Dikensoy, P. Yildiz Gulhan, I. Basyigit, H. Boyaci, I. Oguzulgen, S. Borekci, B. Gemicioglu, F. Bayraktar, O. Elbek et al., The association of antiviral drugs with COVID-19 morbidity: The retrospective analysis of a nationwide COVID-19 cohort Aug 2022, Frontiers in Medicine, Volume 9
LATE TREATMENT 1,472 patient HCQ late treatment study: 112% higher ventilation (p=0.21), 53% higher ICU admission (p=0.33), and 17% longer hospitalization (p=0.05).
Retrospective 1,472 hospitalized patients in Turkey, showing a higher risk of ICU admission and ventilation with HCQ, without statistical significance. https://c19p.org/babayigith
401. O. Babalola, Y. Ndanusa, A. Ajayi, J. Ogedengbe, Y. Thairu, and O. Omede, A Randomized Controlled Trial of Ivermectin Monotherapy Versus Hydroxychloroquine, Ivermectin, and Azithromycin Combination Therapy in Covid-19 Patients in Nigeria Sep 2021, J. Infectious Diseases and Epidemiology, Volume 7, Issue 10
LATE TREATMENT 60 patient HCQ late treatment RCT: 55% lower hospital discharge (p=0.2) and 10% improved viral clearance (p=0.78).
Small RCT with 61 patients in Nigeria, all patients treated with ivermectin, zinc, and vitamin C, showing no significant improvements in recovery with the addition of HCQ+AZ. https://c19p.org/babalola2h
402. F. Syed, M. Hassan, M. Arif, S. Batool, R. Niazi, U. Laila, S. Ashraf, and J. Arshad, Pre-exposure Prophylaxis With Various Doses of Hydroxychloroquine Among Healthcare Personnel With High-Risk Exposure to COVID-19: A Randomized Controlled Trial May 2021, Cureus
101 patient HCQ prophylaxis RCT: 60% more symptomatic cases (p=0.41) and 92% more cases (p=0.12).
Small PrEP RCT of low risk healthcare workers, showing no significant differences. Authors report that there was no hospitalization, ICU care, or death from COVID-19, however table 3 of the preprint shows severe events labeled as “requiring hospitalization” Symptomatology and disease severity results in tables 3 and 4 appear inconsistent. NCT04359537. https://c19p.org/syed
403. J. Calderón, S. Padmanabhan, F. Salazar, D. Hernández, A. Martínez, C. Ortiz, H. Zerón, Treatment with hydroxychloroquine vs nitazoxanide in patients with COVID-19: brief report Nov 2021, PAMJ – Clinical Medicine
LATE TREATMENT 44 patient HCQ late treatment study: 215% higher mortality (p=0.38), 652% higher ventilation (p=0.15), 145% higher ICU admission (p<0.0001), and 107% longer hospitalization (p=0.007).
Planned RCT of HCQ vs. HCQ+nitazoxanide which was aborted due to the now retracted Surgisphere paper written by disgraced, now former physician Sapan Desai. Authors retrospectively analyze a small set of HCQ vs. nitazoxanide patients (which were protocol deviations in the planned RCT), showing reduced hospitalization time and ICU admission with nitazoxanide. https://c19p.org/calderon2h
404. C. Rodrigues, R. Freitas-Santos, J. Levi, A. Senerchia, A. Lopes, S. Santos, R. Siciliano, and L. Pierrotti, Hydroxychloroquine plus azithromycin early treatment of mild COVID-19 in outpatient setting: a randomized, double-blinded, placebo-controlled clinical trial evaluating viral clearance Aug 2021, Int. J. Antimicrobial Agents, Volume 58, Issue 5, Page 106428
EARLY TREATMENT 84 patient HCQ early treatment RCT: 14% improved viral clearance (p=0.15).
RCT 84 low risk patients, 42 treated with HCQ/AZ, showing no significant differences. There was only one hospitalization which was in the treatment arm. https://c19p.org/rodrigues
405. A. Llanos-Cuentas, A. Schwalb, J. Quintana, B. Delfin, F. Alvarez, C. Ugarte-Gil, R. Guerra Gronerth, A. Lucchetti, M. Grogl, and E. Gotuzzo, Hydroxychloroquine to prevent SARS-CoV-2 infection among healthcare workers: early termination of a phase 3, randomised, open-label, controlled clinical trial Feb 2023, BMC Research Notes, Volume 16, Issue 1
68 patient HCQ prophylaxis RCT: 69% more cases (p=0.46).
Early terminated healthcare worker PrEP RCT with only 68 patients and 8 cases, showing no significant difference with HCQ. No information on symptoms per group, case severity, or the timing of cases is provided. https://c19p.org/llanoscuentas
406. S. Florescu, D. Stanciu, M. Zaharia, A. Kosa, D. Codreanu, A. Kidwai, S. Masood, C. Kaye, A. Coutts, L. MacKay, C. Summers, P. Polgarova, N. Farahi, E. Fox, S. McWilliam, D. Hawcutt, L. Rad, L. O’Malley, J. Whitbread, D. Jones, R. Dore, P. Saunderson, O. Kelsall, N. Cowley, L. Wild, J. Thrush, H. Wood, K. Austin, J. Bélteczki, I. Magyar, Á. Fazekas, S. Kovács, V. Szőke, A. Donnelly, M. Kelly, N. Smyth, S. O’Kane, D. McClintock, M. Warnock, R. Campbell, E. McCallion, A. Azaiz, C. Charron, M. Godement, G. Geri, A. Vieillard-Baron, P. Johnson, S. McKenna, J. Hanley et al., Long-term (180-Day) Outcomes in Critically Ill Patients With COVID-19 in the REMAP-CAP Randomized Clinical Trial Dec 2022, JAMA
LATE TREATMENT 352 patient HCQ ICU RCT: 51% higher mortality (p=0.06).
Long-term follow up for the REMAP-CAP very late stage ICU trial, showing higher risk with HCQ, not quite reaching statistical significance. https://c19p.org/higgins
407. A. Barratt-Due, I. Olsen, K. Nezvalova-Henriksen, T. Kåsine, F. Lund-Johansen, H. Hoel, A. Holten, A. Tveita, A. Mathiessen, M. Haugli, R. Eiken, A. Kildal, Å. Berg, A. Johannessen, L. Heggelund, T. Dahl, K. Skåra, P. Mielnik, L. Le, L. Thoresen, G. Ernst, D. Hoff, H. Skudal, B. Kittang, R. Olsen, B. Tholin, C. Ystrøm, N. Skei, T. Tran, S. Dudman, J. Andersen, R. Hannula, O. Dalgard, A. Finbråten, K. Tonby, B. Blomberg, S. Aballi, C. Fladeby, A. Steffensen, F. Müller, A. Dyrhol-Riise, M. Trøseid, and P. Aukrust, Evaluation of the Effects of Remdesivir and Hydroxychloroquine on Viral Clearance in COVID-19 Jul 2021, Annals of Internal Medicine, Volume 174, Issue 9, Page 1261-1269
LATE TREATMENT 93 patient HCQ late treatment RCT: 120% higher mortality (p=0.35).
Small RCT in Norway with 52 HCQ and 42 remdesivir patients, showing no significant differences with treatment. Add-on trial to WHO SOLIDARITY. NCT04321616. https://c19p.org/barratdue
408. I. Schwartz, M. Boesen, G. Cerchiaro, C. Doram, B. Edwards, A. Ganesh, J. Greenfield, S. Jamieson, V. Karnik, C. Kenney, R. Lim, B. Menon, K. Mponponsuo, S. Rathwell, K. Ryckborst, B. Stewart, M. Yaskina, L. Metz, L. Richer, and M. Hill, Assessing the efficacy and safety of hydroxychloroquine as outpatient treatment of COVID-19: a randomized controlled trial Jun 2021, CMAJ Open, Volume 9, Issue 2, Page E693-E702
LATE TREATMENT 179 patient HCQ late treatment RCT: 37% improved recovery (p=0.15).
Small early terminated late treatment RCT showing no significant differences. The HCQ group was a median of 7 days from symptom onset at baseline, which may not include the delay delivering the medication. From the 4 HCQ hospitalizations, only one is in the per-protocol analysis, and that patient was hospitalized one day after randomization (authors do not specify if the patient received and took any HCQ before the hospitalization). The trial was terminated early due to the fraudulent Lancet article (wording here is notably different between the submitted and published versions). Per-protocol analysis, the submitted version, and the peer-review comments (two reviewers, only one with substantial feedback) are in the supplementary material. When a patient reported a symptom, they were asked whether they were still experiencing that symptom, and to choose between these three options when comparing the symptom to their pre-COVID-19 state: (1) “Yes, this problem remains the same”; (2) “Yes, but there’s been SOME improvement”; or (3) “No, this is back to normal.” The patient was classified as having “no improvement” at 1-year if they reported ≥1 symptom at both visits, for which they indicated that the problem remained the same at 1-year. Persistence refers to patients reporting ≥1 symptom that emerged post-COVID-19 and was still present at the time of assessment. For presence of symptoms, the patient reported ≥1 symptom that emerged with or after their COVID-19 infection at some point prior to the time of assessment. https://c19p.org/schwartz2
409. Á. Réa-Neto, R. Bernardelli, B. Câmara, F. Reese, M. Queiroga, and M. Oliveira, An open-label randomized controlled trial evaluating the efficacy of chloroquine/hydroxychloroquine in severe COVID-19 patients Apr 2021, Scientific Reports, Volume 11, Issue 1
LATE TREATMENT 105 patient HCQ late treatment RCT: 57% higher mortality (p=0.2), 115% higher ventilation (p=0.03), and 147% worse recovery (p=0.02).
Early terminated very late stage (99% on oxygen, 81% in ICU, 18% on mechanical ventilation at baseline) RCT with 24 CQ patients, 29 HCQ, and 52 control patients, showing worse clinical outcomes with treatment. NCT04420247. https://c19p.org/reanato
Join the conversation:


Published under a Creative Commons Attribution 4.0 International License
For reprints, please set the canonical link back to the original Brownstone Institute Article and Author.









