Today is the first day of the multi-million pound COVID-19 inquiry set up to ‘examine the UK’s response to and impact of the COVID-19 pandemic, and learn lessons for the future.’
The irony is that one of the most important questions centred around the UK’s COVID-19 response will probably never be asked by the inquiry committee- why the UK Government wasted close to £4 billion on the infamous Innova lateral flow (rapid antigen) test- at a colossal cost to the UK taxpayer.
The further irony, according to the Telegraph, is that ‘the COVID-19 inquiry is demanding that the attendees test for the virus more than a year after the Government scrapped lateral flow tests.’
From June-September 2021, I wrote an investigative report for the TCW (which ended up as a 6-part series) on the US-supplied and Chinese-made Innova COVID-19 test.
Innova Medical Group (IMG), the US company which greatly benefited from the UK Government’s mammoth testing contract, is owned by the private equity group Pasaca Capital, which was founded by a Chinese investment banker, the enigmatic Charles Huang, who has been embroiled in scandal in the past.
The Innova rapid antigen test was seen from the very beginning as the ‘golden child’ of rapid antigen tests and was given special treatment by being warp-speed-tracked through the MHRA’s (UK’s drug and medical device regulator) approval process in 2020.
Forty different lateral flow devices had been put forward. Nine met the criteria to continue to full evaluation, six made it to the third phase, but only the Innova SARS-CoV-2 Antigen Rapid Qualitative Test, the test that had been used in a Liverpool pilot scheme, with a specificity recorded at an alleged, ‘99.68 per cent,’ won through.
How did this fledgling California start-up get so far ahead of the game in being able to supply vast quantities of tests, not only to the UK but also delivering ‘over 500 million Innova SARS-CoV-2 rapid antigen test kits to over 20 countries’ -with the help of the UK Government’s lucrative contracts?
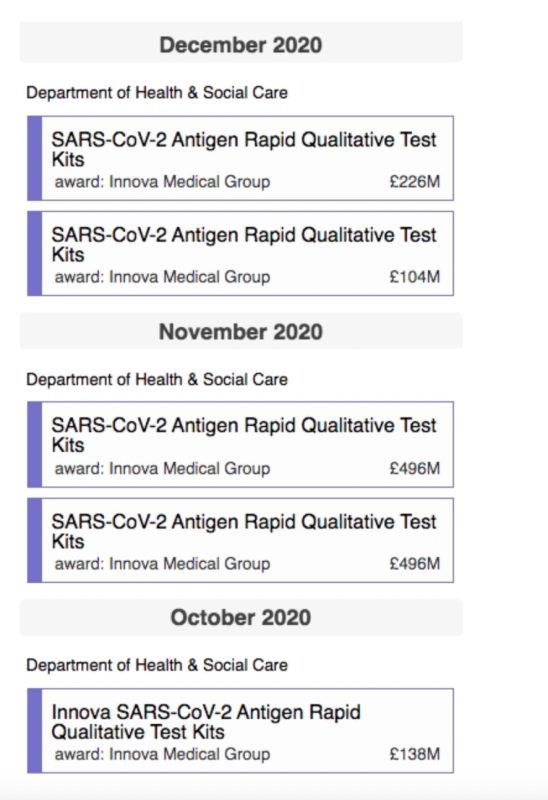
The screenshot below shows some of the early contracts in 2020 between the UK Government and Innova Medical Group, under its discredited NHS Test and Trace scheme, which ended up costing a staggering £32 billion.

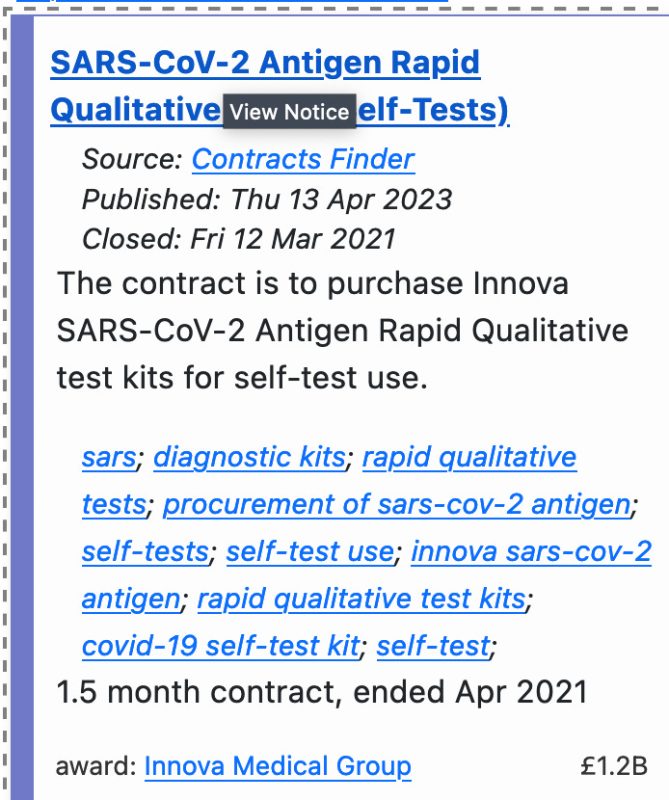
In April 2023, the UK public sector contract site, BidStats, published an unbelievable 2021 contract covering a 1.5 month period, worth a staggering £1.2 billion that was signed by the Department of Health and Social Care for the procurement of Innova’s rapid antigen tests.

Thanks to the UK taxpayer, in 2020 ‘[IMG] executives at the Pasadena startup began flying from Burbank to destinations around the world on a pair of newly registered Gulfstream jets, one a G650 decked out in white plush seats, burnished interiors and other luxury finishes,’ according to the LA Times.
It’s noteworthy that IMG’s corporation filing date was March 27, 2020, right around the time the World Health Organisation announced the SARS-CoV-2 virus a ‘global pandemic.’
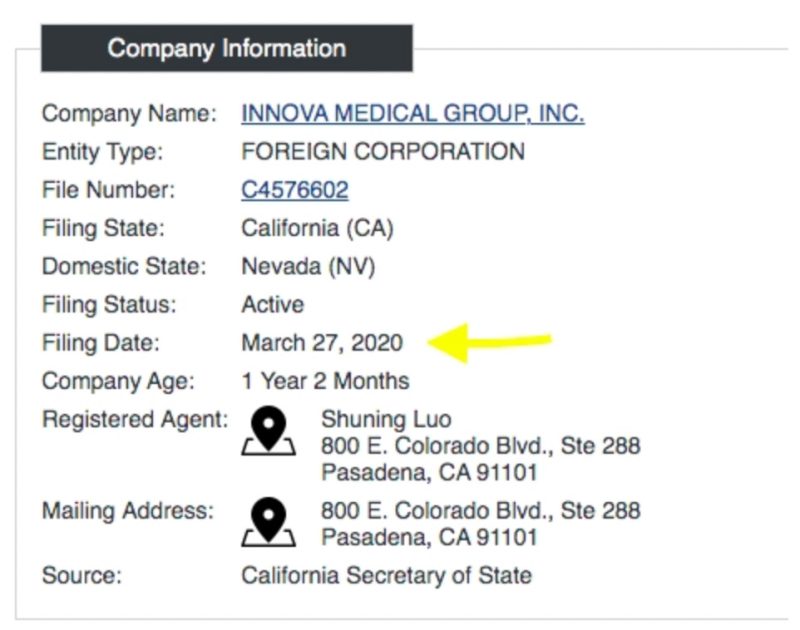

The corporation’s domestic state is listed as Nevada, which happens to have the most personal liability protection for directors over corporate wrongdoing and prevents creditors from pursuing companies’ assets.
IMG’s website states that after Huang set up IMG in March 2020, ‘the Innova team, together with its primary contract manufacturer, Xiamen Biotime Biotechnology Co Ltd, based in Fujian, China, spent several months designing a highly accurate rapid antigen test for Covid-19.’
Below is the ‘Qualification Certificate’ that was found inside the NHS Test and Trace Covid-19 self-test kits.


As part of its exclusive global rights to the mass-produced rapid antigen tests made by its ‘partner’ Xiamen Biotime Biotechnology in China, IMG made itself the UK’s sole supplier.
In June 2021, after the UK Government had spent billions on this infamous test (and after IMG execs had purchased several more private jets and luxury homes), the US Food and Drug Administration (FDA) issued a warning to the public: ‘Stop Using Innova Medical Group SARS-CoV-2 Antigen Rapid Qualitative Test’ and urged them ‘to place them in the trash.’
Why? These tests were proven to be ‘not fit for purpose’ with the FDA having significant concerns about their performance, which had not been adequately established. In addition, ‘labelling distributed with certain configurations of the test includes performance claims that did not accurately reflect the performance estimates observed during the clinical studies of the tests.’
What is truly remarkable is that the FDA’s Class I recall of the Innova test was completely ignored by the UK Government and the MHRA. In fact, they double-downed and went on to sign more contracts with IMG, of course all paid for by the UK taxpayer.
My 6-part investigative series published in TCW, Defending Freedom, on the scandals of the Innova COVID-19 test can be read in further detail by clicking on the links: Part 1 | Part 2 | Part 3 | Part 4 | Part 5 | Part 6
Reprinted from the author’s Substack
Join the conversation:


Published under a Creative Commons Attribution 4.0 International License
For reprints, please set the canonical link back to the original Brownstone Institute Article and Author.









