Part 1 of my analysis of the Periodic Safety Update Report #3 (PSUR #3) for the Pfizer-BioNTech Covid-19 mRNA vaccine, covering the 6-month period of 19 December 2021 through to 18 June 2022, focused on the disturbing pregnancy and lactation cases. Part 2 of the report focuses on the tragic child deaths.
Firstly, a comparative look at the data in PSUR #3 revealed similar findings in 1st PSUR, apart from a significant 55% increase in the number of case reports and a 36% increase in the number of adverse events recorded. The following similarities were found in both sets of data: three times the number of cases were reported for women; the age group most affected was for 31-50-year-olds; one-third of all cases were classified as serious and a significantly high percentage of cases were classified with outcomes as either unknown or not recovered.
An Overview of the Data
- 508,351 cases (individuals) suffering from 1,597,673 adverse events
- Three times the number of cases were reported for women than men
- 1/3 of all cases were classified as serious
- 3,280 deaths reported
- 60% of cases were reported with either outcome unknown or not recovered
- 92% of cases did not have any comorbidities
- Highest number of cases occurred in the 31-50 year age group
- Germany had the highest recorded number of cases (22.5% of all reported worldwide
- cases)
Germany had the highest number of recorded cases, a total of 114,573, which accounted for 22.5% of all worldwide cases for that 6-month period. It is worth noting that from December 2020 through to June 2022, a staggering 323,684 individual reports of suspected Covid-19 vaccine side effects were received by The Paul-Ehrlich-Institut, an Agency of the German Federal Ministry of Health. Yet, despite this large number, Karl Lauterbach, Germany’s Minister for Health, known for his pro-lockdown and pro-vaccine stance, made the unsubstantiated claim that the “vaccine was without side effects” in August 2021.
However, earlier this year, Lauterbach made a surprising U-turn in a TV interview, where he stated, “These unfortunate cases [of Covid-19 vaccine adverse effects] are heart-breaking and every victim is one too many…” Only recently, the first lawsuit against BioNTech was filed in Germany, by the law firm, Rogert and Ulbrich, with the plaintiff seeking damages due to an injury, allegedly caused by the German vaccine manufacturer’s mRNA product.
The Paediatric Cases (Less Than or Equal to 17 Years)
In the pharmacovigilance documents, PSUR #1 and #3, paediatric cases were identified as cases where the age for the patient was ‘less than or equal to 17 years.’ In PSUR #1, a shocking disclaimer is made from the onset, which can be seen in the screenshot below.
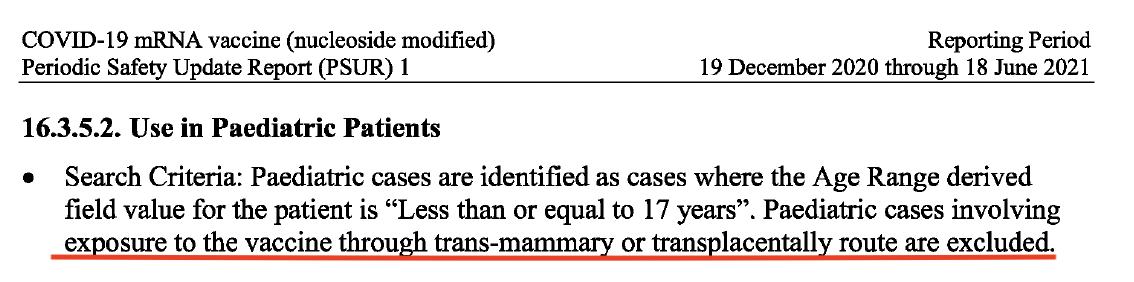

Part 1 of this report detailed how both Pfizer and BioNTech, along with the European Medicines Agency (EMA) and the US. Food and Drug Administration (FDA) knew the mRNA vaccine crossed the placenta and entered the breast milk, from early 2021. The following statement, ‘paediatric cases involving exposure to the vaccine through trans-mammary or transplacentally route are excluded’, does not only serve as an admission of this fact but more importantly points to a grave abdication of responsibility in protecting infants and the unborn, by Pfizer and BioNTech’s exclusion of these important cases from review, which the EMA shockingly accepted.
In PSUR #3, a staggering 1,843% increase (a total of 31,930 paediatric cases) was observed in the number of individual paediatric cases reporting adverse events, for the first 6 months in 2022, compared to the number of cases reported in PSUR #1 of 1,643 cases, retrieved in the first 6 months of 2021. A contributing factor for this significant increase is the EMA’s approval of the Pfizer-BioNTech vaccine for 12–15-year-olds on May 28, 2021 and for 5-11-year-olds, on November 25, 2021.
The 5–11-Year-Olds
In the post-marketing dataset, there were 9,605 individual cases (almost 2% of total of 507,683 adverse event cases) reported in this age group, compared to 1,227 cases in the previous 2nd PSUR, which covered the last 6 months of 2021. This represents a significant 683% increase. The number of cases retrieved in the previous PSUR was referenced in PSUR #3. At this present time, PSUR #2 has not yet been released by the EMA (even though a FOIA request was made). The US had the highest number of reported cases, followed by Australia, the Philippines, and Germany. Approximately 17% of the total adverse event number of 22,457 were classified as serious. However, what is more concerning is that just over 40% of all adverse events were classified as having an ‘unknown outcome’ and 14.5% as ‘not resolved.’
Disturbingly, there were 20 deaths recorded for this age group. A screenshot below shows the most frequently reported preferred terms (PTs) in the fatal cases.


Out of the 20 deaths, 2 were attributed to myocarditis (inflammation of the heart muscle). Their cases are detailed in the screenshot below.


Both these children suffered from cardio-respiratory arrest which resulted in their death and both developed myocarditis soon after vaccine dose 1 or 2 was administered.
In the case of the 6-year-old boy who died, ‘the reporter concluded that the death “had nothing to do” with the administration of BNT162b2 and was due to natural causes.’
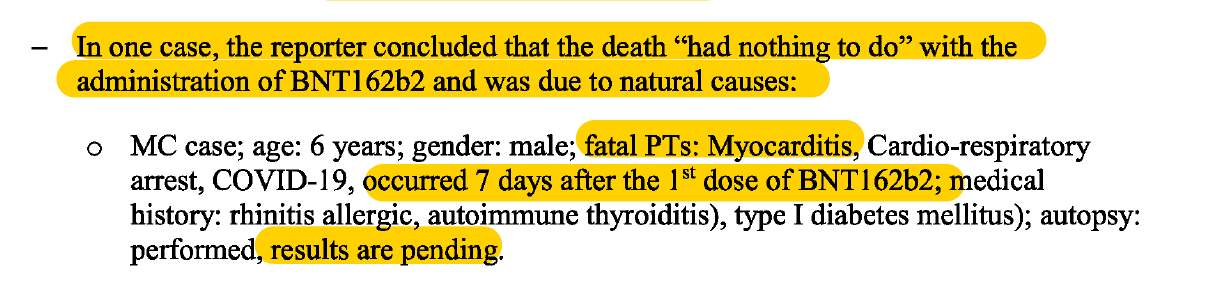

For the ‘reporter’ to completely rule out that the child’s death had “nothing to do” with the vaccine shows undeniable bias, considering that the fatal events of Myocarditis and Cardio-respiratory arrest, occurred 7 days after the 1st dose was administered. Furthermore, how could such a conclusion be reached when the results of the autopsy were still pending?
Myocarditis is an identified known risk of the mRNA Covid-19 vaccines. On June 11, 2021, the EMA’s safety committee, the Pharmacovigilance Risk Assessment Committee (PRAC), issued a statement acknowledging myocarditis and pericarditis as possible side effects of Covid-19 mRNA vaccines. A January 2022 study by Oster et al., published in JAMA, revealed that ‘the risk of myocarditis after receiving mRNA-based Covid-19 vaccines was increased across multiple age and sex strata.’
What is noteworthy is that in the EMA’s PRAC assessment report on PSUR #3, which was also released by the Freedom of Information Act, the Rapporteur (Menno van der Elst) criticised how these 2 fatal myocarditis cases (medically confirmed) were only “briefly described.” Failure by the MAH (Marketing Authorisation Holder), in this case, BioNTech Manufacturing GmbH, to provide an assessment “according to the Brighton Collaboration Myocarditis case definition and level of certainty classification” along with a “WHO causality assessment per case regarding Comirnaty [marketing name for Pfizer-BioNTech mRNA vaccine] exposure” was deemed unacceptable. (See screenshot below)
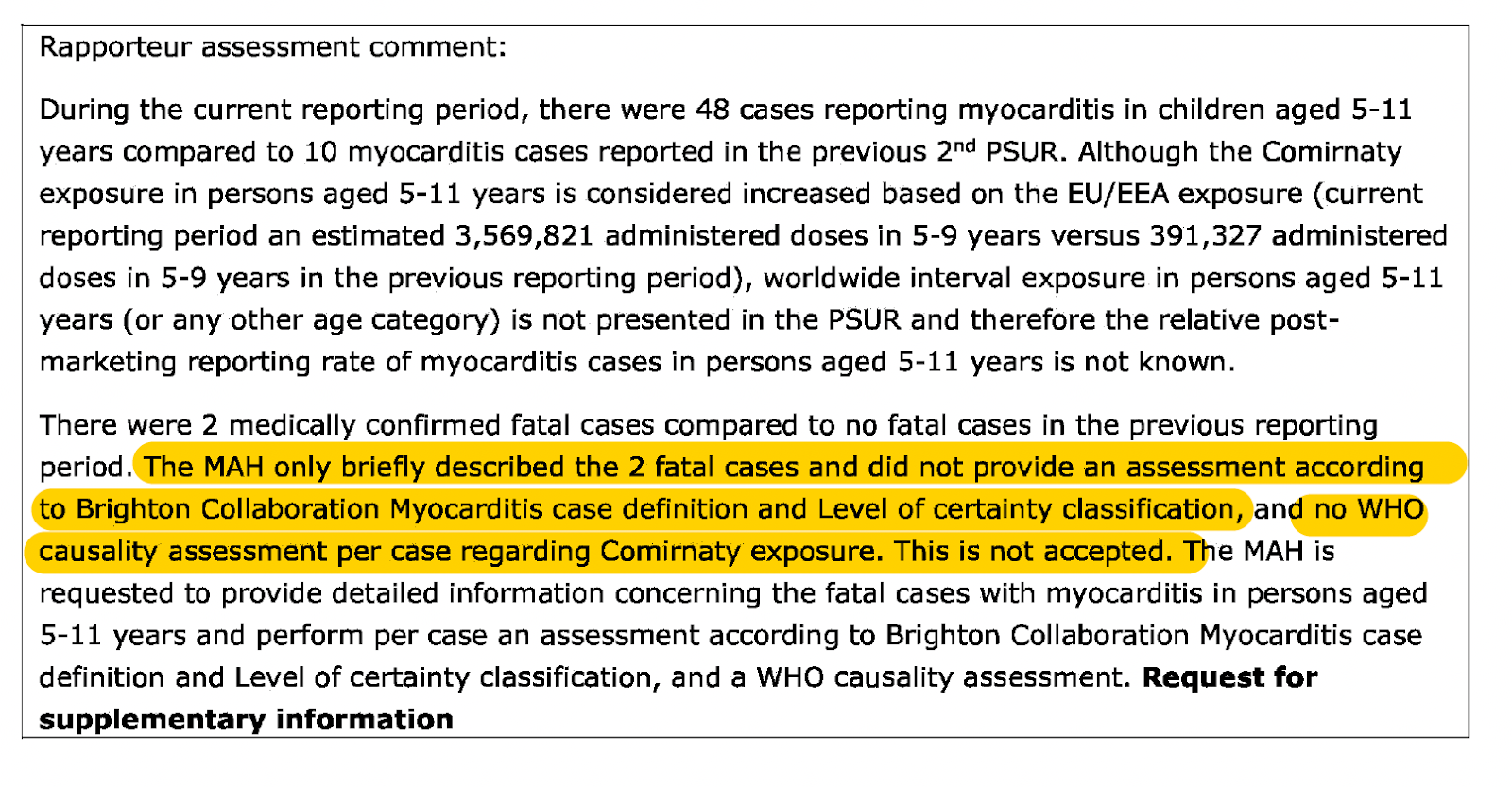

In addition, the Rapporteur reveals the fact that 48 cases of children aged 5-11 reported myocarditis compared to 10 in the previous 2nd PSUR. This is then followed up with the comment that the post-marketing reporting rate of myocarditis cases in this age group is not known.
Two further fatal cases were outright dismissed since ‘death’ was reported as the only fatal adverse event. Based on this fact alone, ‘limited information prevented any meaningful assessment.’
Two fatal cases were dismissed because the children had underlying medical conditions – even though the autopsies were either reported as ‘not performed’ or ‘unknown if performed.’
In 1 fatal case, a 6-year-old boy’s death was ruled out as having anything to do with the vaccine, even though he developed myocarditis, 7 days after the first dose. (This case was mentioned earlier in this report.)
A breakdown of the remaining 15 fatal cases can be seen in the screenshot below.
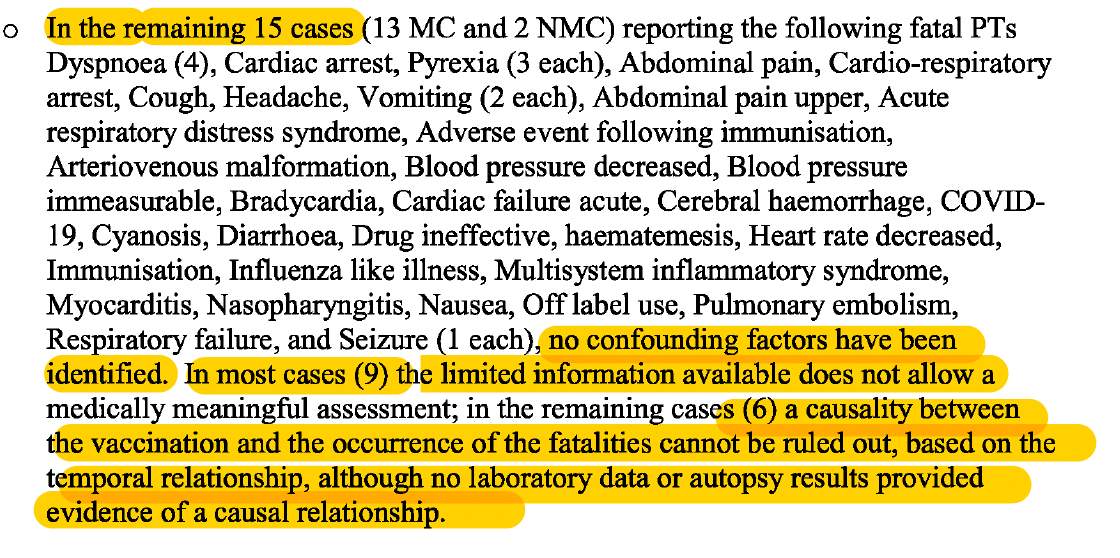

It is interesting that ‘no confounding factors’ were identified for these fatal cases. A confounding factor is one that may distort or mask the effects of another variable, such as an underlying medical condition. This implies that the fatal events listed above could have been attributed to the vaccine. What is also striking is that the report states ‘in the remaining cases (6) a causality between the vaccination and the occurrence of the fatalities cannot be ruled out, based on the temporal relationship, although no laboratory data or autopsy results provided evidence of a causal relationship.’
The 12-17-Year-Olds
There were 21,945 individual cases (4.3% of the 507,683 cases, the total post-marketing dataset) reporting 61,071 adverse events in this age group. PSUR #3 revealed that 18,451 cases were retrieved in PSUR #2, representing an increase of roughly 20% in case numbers. Germany had the highest number of cases reported, closely followed by the Philippines and Australia.
Disturbingly, roughly one-third (32%) of all adverse events were classified as serious; another third as outcome ‘unknown’ and one-fifth (20%) as ‘not resolved.’ The fact that a significant proportion of adverse events (symptoms) experienced by 12-17-year-olds were serious; had outcomes as either unknown or not resolved (had symptoms they did not recover from) by the end of the reporting interval, is particularly damning.
Tragically, 62 fatal cases were reported for this age group, roughly three times more than in the 5-11-year-old age group. A breakdown of the fatal cases by age, can be seen below.
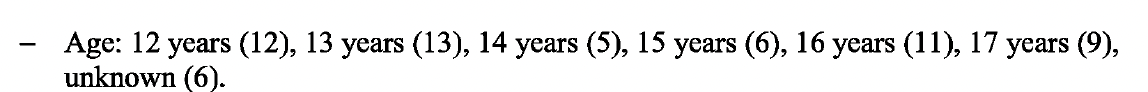
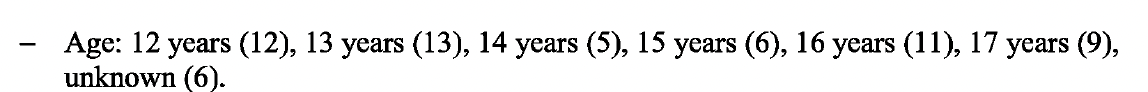
The most frequently reported fatal preferred terms can also be seen below.


It’s noteworthy that the fatal preferred terms (PTs): Myocarditis, cardiac arrest, and dyspnoea (shortness of breath) were also observed in the younger age group.
In the three fatal myocarditis cases, all were aged 13 years. The brief case details can be read below.


For two out of the three fatal myocarditis cases, it was ‘not reported if an autopsy was performed.’ In the autopsy that was done for the 13-year-old girl who died, ‘Adverse event following immunisation’ are the only details given.
The following is a breakdown of the 62 fatal cases, summarised below:
In 15 cases, ‘Death’ was the only reported adverse event, therefore according to the PSUR ‘limited information provided any meaningful assessment.’
In 2 cases the subjects did not die due to illness but ‘unfortunate accidents.’
In 6 cases, ‘underlying medical conditions could have predisposed the occurrence of the fatal adverse events.’ In one of these cases, a 16-year-old girl, the adverse events of pulmonary embolism and cardiac arrest, all developed 2 days after the 3rd dose of BNT162b2. An autopsy was performed but the results were ‘not provided.’
The screenshot below shows the details of the remaining 39 cases. The list of fatal adverse events is extensive.


It’s worth noting that no confounding factors were identified in all 39 cases. The fact that 19 were simply written off due ‘limited information available’ shows complete indifference and inaction on behalf of Pfizer and BioNTech by not bothering to investigate these deaths, or even attempt to seek out further information.
For the remaining 20 cases, the report states, ‘causality between the vaccination and the occurrence of the fatalities cannot be ruled out, based on the temporal relationship, although no laboratory data or autopsy results provided evidence of a causal relationship.’
This carefully worded statement by Pfizer/BioNTech does not deny the possibility that vaccination was the cause of death but hides behind the lack of ‘evidence of a causal relationship’, rendering it moot.
This FOIA (Freedom of Information Act) released pharmacovigilance document reveals that for these fatal cases, autopsies were either simply not done; unknown if done or the reason, ‘limited information was provided’ is given.
This lack of investigation into these child/adolescent deaths is egregious, particularly since it is highly unusual for a young person to experience either a pulmonary embolism, cardiac arrest, myocarditis, ruptured aneurysm, cardiac failure, to name but a few of the devastating adverse events resulting in death.
Dr Clare Craig, a UK-based diagnostic pathologist, spoke exclusively to Children’s Health Defense, Europe about the issue of autopsies and vaccination: ‘There is a Catch-22. A series of post-mortems demonstrating the mechanisms of action of the vaccine could be used as evidence of a mechanism, but pathologists largely won’t make the connection in an individual until they’ve seen such a series published. The tail wags the dog.’
In the same way, no doctors certified any deaths caused by brain clots until the MHRA [the UK’s Medicines and Healthcare products Regulatory Agency] said it was an issue based on Scandinavian data. Then doctors started putting it as a cause.
The situation is not helped by a culture of not investigating deaths by post-mortem. Any unexpected death especially in a child should have a post-mortem in my view. What’s more, pathologists do not always take tissue samples to enable microscopic diagnosis.’
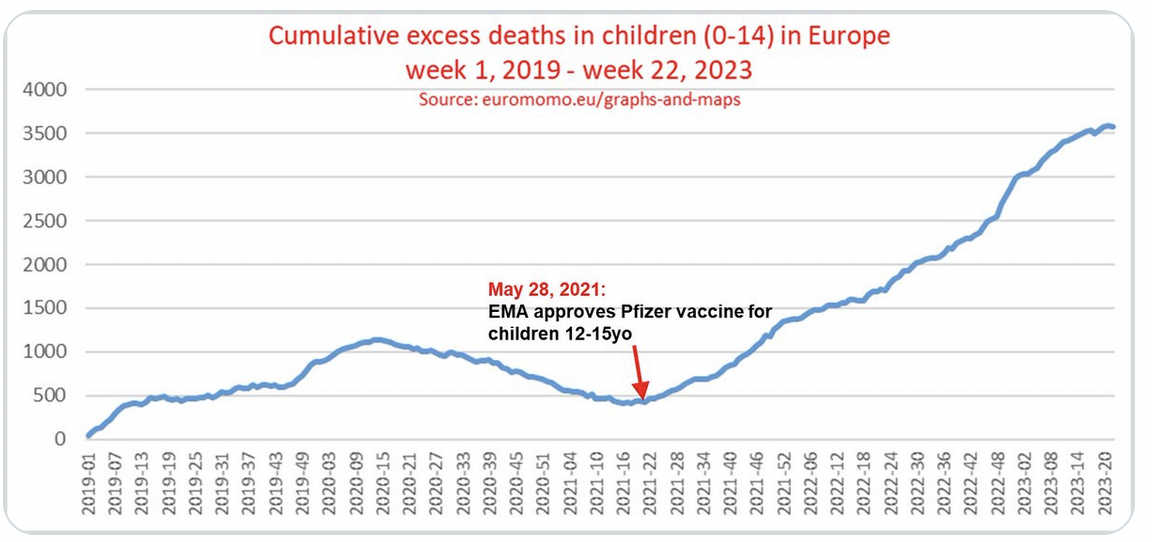
EuroMOMO, which graphs pooled weekly total number of deaths in the data provided by EuroMOMO partner countries, showed cumulative excess deaths in children aged 0-14 years in Europe, from week 1, 2019- week 22, 2023. A significant increase can be observed, just around the time the EMA approved the Pfizer-BioNTech Covid-19 vaccine for children aged 12-15 years of age.

Turning to the EMA’s PRAC assessment of PSUR#3, their overview of paediatric deaths for both age groups (resulting in 79 cases, 3 cases out of the 82 were non-fatal) can be viewed in the screenshot below.


It’s not surprising that the MAH (BioNTech) conclude, ‘No information in the review of these 82 paediatric fatal cases that identifies BNT162b2 as a contributor to the reported deaths.’
Their disingenuous statement, ‘Fatal cases will continue to be monitored via routine pharmacovigilance’ can be interpreted as their get-out clause.
In the EMA’s Rapporteur comment box, common phrases and terms describing these fatal cases can be read throughout the report, such as, ‘unlikely related to Comirnaty exposure’, ‘unclassified’, and ‘unassessable.’
What is altogether shocking and unconscionable is the EMA’s endorsement of the MAH’s conclusion…and their signing off with ‘issue solved.’ If only the issue could be that easily ‘solved’ for the family members tragically left behind.
Recently, only a few of the PSUR appendices were released via FOIA request, one of them was Appendix 6C.2 Cumulative Summary Tabulation of Fatal Reports.

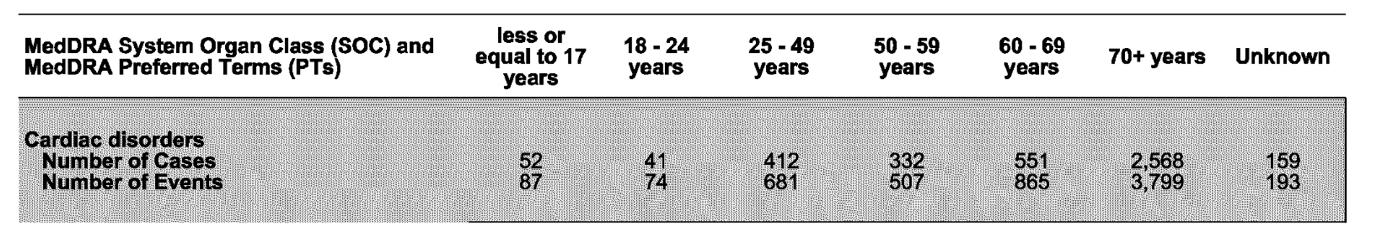
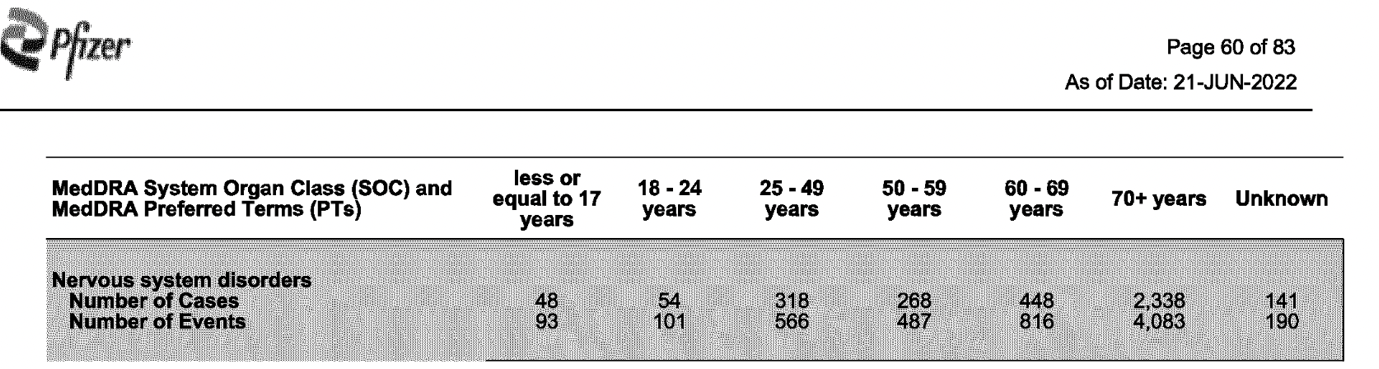
According to Pfizer’s 83-page report of fatal cases, a total of 13,659 deaths (all age groups) were retrieved from 21 December 2020 through to 18 June 2022.
It’s noteworthy that the highest number of fatal cases, reported across all age groups, were classified under Cardiac and Nervous system disorders.


Interviews
Republished from Children’s Health Defense Europe
Join the conversation:


Published under a Creative Commons Attribution 4.0 International License
For reprints, please set the canonical link back to the original Brownstone Institute Article and Author.