An increasing number of prominent medical professionals have called for an immediate halt in the use of the alleged, “safe and effective” COVID-19 mRNA shots. Now, the Florida State Surgeon General, Dr. Joseph Ladapo, has joined the ever-growing list.
An excerpt from the Florida Department of Health’s January 3 bulletin reads:
The Surgeon General outlined concerns regarding nucleic acid contaminants in the approved Pfizer and Moderna COVID-19 mRNA vaccines, particularly in the presence of lipid nanoparticle complexes, and Simian Virus 40 (SV40) promoter/enhancer DNA. Lipid nanoparticles are an efficient vehicle for delivery of the mRNA in the COVID-19 vaccines into human cells and may therefore be an equally efficient vehicle for delivering contaminant DNA into human cells. The presence of SV40 promoter/enhancer DNA may also pose a unique and heightened risk of DNA integration into human cells.
The bulletin included the following statement from Dr. Ladapo:
DNA integration poses a unique and elevated risk to human health and to the integrity of the human genome, including the risk that DNA integrated into sperm or egg gametes could be passed onto offspring of mRNA COVID-19 vaccine recipients. If the risks of DNA integration have not been assessed for mRNA COVID-19 vaccines, these vaccines are not appropriate for use in human beings.
The Florida Surgeon General’s warnings centred around the potential health risks associated with insertional mutagenesis (the integration of foreign DNA with the subject/host’s genome). But he did not stop there – he also raised a further concerning risk of generational mutagenesis. Where the “DNA integrated into sperm or egg gametes could be passed onto the offspring of mRNA COVID-19 vaccine recipients.”
The basis of Dr Ladapo’s warning regarding the presence of the “SV40 promoter/enhancer DNA” in the mRNA shots is founded on the bombshell findings of a recent pre-print study. Significant levels (billions to hundreds of billions of fragments of plasmid DNA) were found in both Moderna and Pfizer/BioNTech’s monovalent and bivalent mRNA shots, vastly exceeding regulatory standards. Leading one to conclude that the injectable mRNA products that have gone into the arms of billions are heavily adulterated, as well as being misbranded.
The study’s lead author, molecular virologist, Dr David J. Speicher offered me his response to Dr. Ladapo’s statement.
It is encouraging to see the courageous stand by Dr. Joseph Ladapo to call for a halt to the COVID-19 vaccines, and an honour to know that our study was instrumental in making that decision. Our Canadian study confirmed previous reports of Kevin McKernan and Dr. Philip Buckhaultz, which showed high levels of residual plasmid DNA within the lipid nanoparticles in the COVID-19 modRNA vaccines. The SV40 enhancer-promoter in the Pfizer vaccines is a risk for genomic integration as well as interfering with the p53 gene (the guardian of the genome). Both could drive an increase risk for cancer. Our data on the adulteration coupled with Mulroney et al’s Nature paper showing an aberrant immune response due to ribosomal frameshifting should be sufficient data for these vaccines to be halted globally. We need immediate research into the effects of these vaccines on human health. I applaud Dr. Ladapo for his stance and hope that other places, like Alberta, will follow suit.
In stark contrast to Speicher’s praise of Ladapo, Dr Paul Offit, member of the Food and Drug Administration (FDA) Vaccines and Related Biological Products Advisory Committee, responsible for the ‘rubber stamping’ of the COVID-19 mRNA injections, said in an interview: “It is hard to believe that Dr. Ladapo actually issued that statement” and went on to dismiss all of the Florida Surgeon General’s concerns.
A month prior, the FDA responded to a letter sent by Dr Ladapo stating, “with over a billion doses of the mRNA vaccines administered, no safety concerns related to residual DNA have been identified.”
The FDA letter was written by Dr Peter Marks, Director of Center for Biologics Evaluation and Research (CBER), who also dismissed Ladapo’s claims as “quite implausible” and “misleading.”
However, what is impossible for the FDA officials to deny is that the mRNA shots are an even more experimental form of gene therapy.
They Were Never Vaccines
Despite being marketed as “vaccines” to promote uptake, both the Moderna and Pfizer-BioNTech shots meet the criteria of a gene therapy, as well as going beyond that.
In BioNTech’s US Security and Commission (SEC) Filing, it states, “mRNA is considered a gene therapy product by the FDA.” It goes on to state: “No mRNA immunotherapy has been approved, and none may ever be approved, in this new potential category of therapeutics. mRNA drug development has substantial clinical development and regulatory risks due to the novel and unprecedented nature of this new category of therapeutics.”
Gene therapy has been around for several decades and involves the use of viral vectors, which are modified viruses that deliver therapeutic genes into target cells. However, the mRNA-based shots are in an entirely “novel and unprecedented” class of their own.
Early studies have shown that with gene therapy, serious health risks can occur, leading to toxicity, inflammation, and even cancer. There have been notable cases of leukaemia in early gene therapy trials, which have raised awareness of the risk of insertional mutagenesis.
One would assume, based on the highly experimental nature of the mRNA injectable product, a more stringent and robust safety assessment would have been conducted, before it was rolled out to the public but that was not the case.
My investigative reports for Trial Site News, written nearly two years ago, on the trove of Pfizer/BioNTech documents (which the FDA wanted to remain hidden from the public until 2096) revealed that critical safety studies relating to genotoxicity and carcinogenicity, were never done, and neither were they deemed “necessary” due to them being treated under the guise of conventional vaccines.
The Novel Ingredients, not Intended for Human Use
Two out of the four compounds comprising the lipid nanoparticles (LNPs) which encapsulate the modified (synthetic) mRNA had never been used before in a medicinal product: ALC-0315 and ALC-0519, both licensed from Acuitas Therapeutics. Acuitas’ LNPs are components of the Pfizer/BioNTech and Moderna mRNA Covid-19 vaccines. Furthermore, scientific literature shows these LNPs can be highly toxic and inflammatory.
During Dr Ryan Cole’s address at the UK parliamentary office, which I reported on, he shared the following.
“In the lipid nanoparticles’ data sheet it states these are not for human and not for veterinary use. These are for research purposes only. Yet, they went into 5 billion people around the world!”
Dr Cole’s full presentation can be watched here.
The Risk of Immunological Events from Aberrant Proteins
Buried in the EMA’s February 2021 assessment report, a concern was raised by the regulator about the “truncated and modified RNA” and the risk that “when present in the cell there is a possibility that aberrant proteins will be expressed with possibilities for unwanted immunological events.”


What is interesting is that the EMA determined this risk to be “low.” However, their concern, which they went on to dismiss, has now been recently confirmed in a landmark study on the Pfizer/BioNTech mRNA shots by Cambridge University. The study showed the occurrence of “ribosomal frameshifting” triggered by the modified/synthetic mRNA, resulting in aberrant “unintended proteins” being expressed along with “unintended” immune responses to it.
Amidst the myriad of red flags raised against these novel ‘vaccines,’ another disturbing yet overlooked fact has become more apparent, as new evidence has come to light. The mass-produced mRNA product rolled out to the public was not the same one that was tested in Pfizer’s clinical trial. It should be noted that it was the clinical trial results announced with global fanfare that the regulators, allegedly, based their approval on.
Pfizer’s “Bait-and-Switch”
A few months ago, I interviewed Joshua Guetzkow PhD., who succinctly explained how Pfizer/BioNTech conducted a “bait-and-switch” of their biological product that went on to be injected into the arms of billions.
A key takeaway from the interview was that the Pfizer/BioNTech mRNA product rolled out to the masses was not the same one tested in Pfizer’s pivotal clinical and non-clinical trials (animal studies).
This is because the commercially rolled out product was made using an entirely different method/process. Guetzkow, categorically stated, when it comes to biological products: “The process is the product.”
The product used in the clinical trial was made via Process 1.
- Small-scale clinical batches made using the more expensive PCR process to amplify the DNA template used to make the modified mRNA for the vaccines.
- A highly efficient filtration mechanism was also employed using magnetic beads.
The product that was sold and distributed around the world was manufactured using Process 2.
- Large-scale batches made using a much cheaper process – E. coli bacteria was selected to replicate the DNA used as a template for the mRNA.
- This introduced contamination both from the residual (plasmid/bacterial) DNA and the E. coli membranes called endotoxins, which are also highly inflammatory.
- The significant drop in RNA integrity (a measure of how intact the RNA molecule is) was a direct result from this switch from Process 1 to Process 2.
The Leaked European Medicines Agency Emails
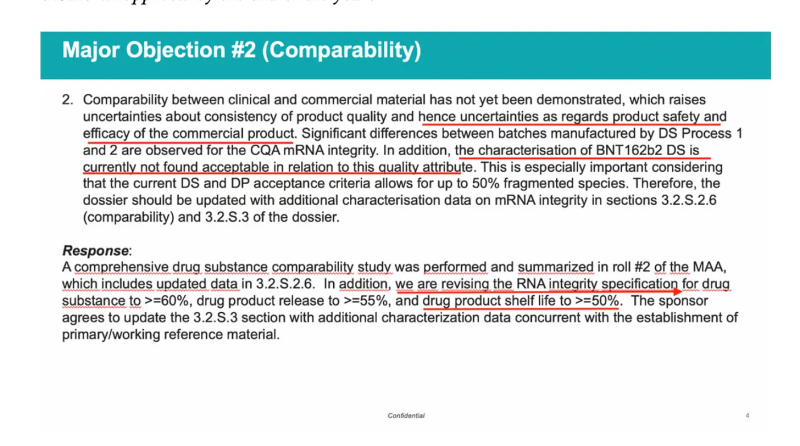
I have extensively written on the leaked EMA emails which first exposed the fact that there was a significant drop in RNA integrity. The private internal email from Evdokia Korakianiti (an EMA scientific administrator) to his colleagues, shown below, highlights this significant drop in %RNA integrity in the proposed commercial batches (made by Process 2) compared to the clinical ones (made by Process 1).


The leaked 26 November 2020 PowerPoint presentation of a meeting between Pfizer-BioNTech and the EMA, shown below, reveals how this major objection was shockingly ‘resolved’ – the RNA integrity specification was simply lowered down to 50%, meaning that up to half of all mRNA molecules in the commercial batches were allowed to be truncated (not intact). More importantly, the potential implications of the RNA integrity loss in terms of safety and efficacy were completely unknown.

The Promised Comparison Study was Never Done
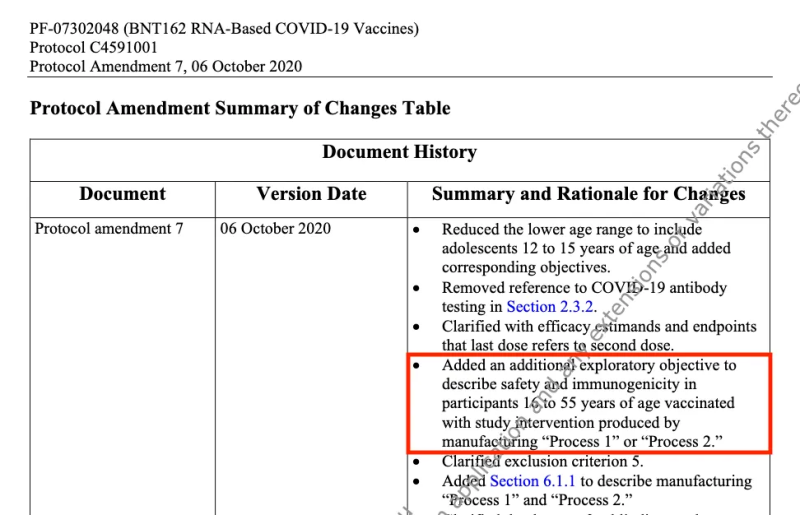
In response to the switch from Process 1 to Process 2 “to support an increased scale of manufacture,” Pfizer added an amendment to its original clinical trial protocol. It promised that it would conduct a comparison study to explore the safety and immunogenicity in individuals 16 to 55 years of age vaccinated with “Process 1” and “Process 2.”


This protocol amendment was made on 6 October 2020.

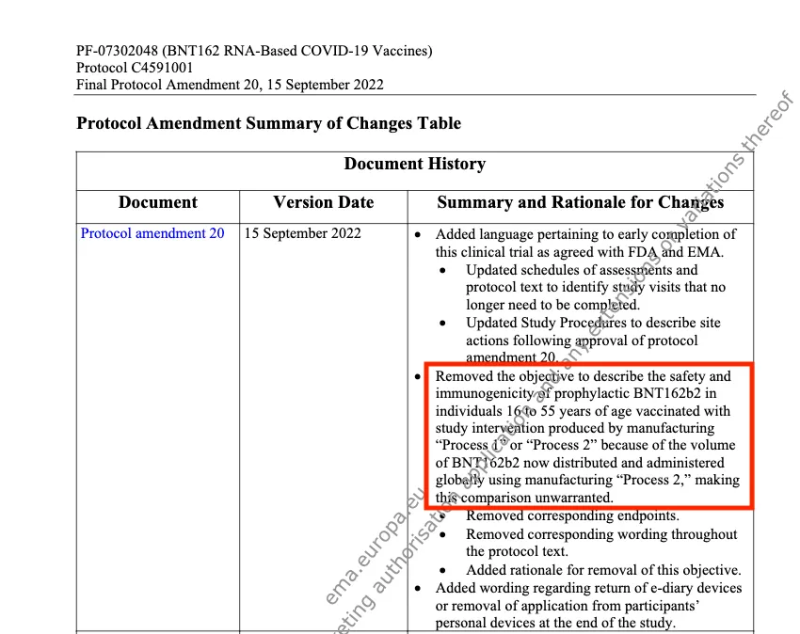
New evidence has come to light, which shows that no comparison study was ever done. On the social media platform X, an anonymous account posted a key section from Pfizer’s “Protocol Amendment 20, 15 September 2022.”
On 15 September 2022, Pfizer removed ‘the objective to describe the safety and immunogenicity of prophylactic BNT162b2 in individuals 16 to 55 years if age vaccinated with study intervention produced by manufacturing “Process 1” or “Process 2” because of the volume of BNT162b2 now distributed and administered globally using manufacturing “Process 2” making the comparison unwarranted.’

Pfizer’s excuse for the study being “unwarranted” because the “Process 2” product had already been rolled out and administered in large volumes globally is deplorable.
Moreover, a plausible conclusion can be drawn: if the “Process 1” product was the one tested on animals (nonclinical trials) and used in the clinical trial on human subjects – then the “Process 2” product, which was rolled out globally, did not even undergo any animal testing, let alone a clinical trial.
This goes far beyond lack of informed consent – when the public unknowingly, have been used as lab rats.
Republished from the author’s Substack
Join the conversation:


Published under a Creative Commons Attribution 4.0 International License
For reprints, please set the canonical link back to the original Brownstone Institute Article and Author.









