As some people have now been vaccinated for more than half a year, evidence is pouring in about Covid vaccine efficacy. The gestalt of the findings implies that the infection explosion globally that we have been experiencing– post double vaccination in e.g. Israel, UK, US etc. –may be due to the vaccinated spreading Covid as much or more than the unvaccinated.
A natural question to ask is whether vaccines with limited capacity to prevent symptomatic disease may drive the evolution of more virulent strains? In a PLoS Biology article from 2015, Read et al. observed that:
“Conventional wisdom is that natural selection will remove highly lethal pathogens if host death greatly reduces transmission. Vaccines that keep hosts alive but still allow transmission could thus allow very virulent strains to circulate in a population.”
Hence, rather than the unvaccinated putting the vaccinated at risk, it could theoretically be the vaccinated that are putting the unvaccinated at risk.
Here I summarize studies and reports that shed light on vaccine induced immunity against Covid. They highlight the problems with vaccine mandates that are currently threatening the jobs of millions of people. They also raise doubts about the arguments for vaccinating children.
| 1) Gazit et al | showed that “SARS-CoV-2-naïve vaccinees had a 13-fold (95% CI, 8-21) increased risk for breakthrough infection with the Delta variant compared to those previously infected.” When adjusting for the time of disease/vaccine, there was a 27-fold increased risk (95% CI, 13-57). |
| 2) Acharya et al. | Ignoring the risk of infection, given that someone was infected, Acharya et al. found “no significant difference in cycle threshold values between vaccinated and unvaccinated, asymptomatic and symptomatic groups infected with SARS-CoV-2 Delta.” |
| 3) Riemersma et al. | found “no difference in viral loads when comparing unvaccinated individuals to those who have vaccine “breakthrough” infections. Furthermore, individuals with vaccine breakthrough infections frequently test positive with viral loads consistent with the ability to shed infectious viruses.” Results indicate that “if vaccinated individuals become infected with the delta variant, they may be sources of SARS-CoV-2 transmission to others.” They reported “low Ct values (<25) in 212 of 310 fully vaccinated (68%) and 246 of 389 (63%) unvaccinated individuals. Testing a subset of these low-Ct samples revealed infectious SARS-CoV-2 in 15 of 17 specimens (88%) from unvaccinated individuals and 37 of 39 (95%) from vaccinated people.” |
| 4) Chemaitelly et al. | In a study from Qatar, Chemaitelly et al. reported vaccine efficacy (Pfizer) against severe and fatal disease, with efficacy in the 85-95% range at least until 24 weeks after the second dose. As a contrast, the efficacy against infection waned down to around 30% at 15-19 weeks after the second dose. |
| 5) Riemersma et al. | From Wisconsin, Riemersma et al. reported that vaccinated individuals who get infected with the Delta variant can transmit SARS-CoV-2 to others. They found an elevated viral load in the unvaccinated and vaccinated symptomatic persons (68% and 69% respectively, 158/232 and 156/225). Moreover, in the asymptomatic persons, they uncovered elevated viral loads (29% and 82% respectively) in the unvaccinated and the vaccinated respectively. This suggests that the vaccinated can be infected, harbor, cultivate, and transmit the virus readily and unknowingly. |
| 6) Subramanian | Subramanian reported that “at the country-level, there appears to be no discernable relationship between percentage of population fully vaccinated and new COVID-19 cases.” When comparing 2947 counties in the United States, there were slightly less cases in more vaccinated locations. In other words, there is no clear discernable relationship . |
| 7) Chau et al. | looked at transmission of SARS-CoV-2 Delta variant among vaccinated healthcare workers in Vietnams. Of 69 healthcare workers that tested positive for SARS-CoV-2, 62 participated in the clinical study, all of whom recovered. For 23 of them, complete-genome sequences were obtained, and all belonged to the Delta variant. “Viral loads of breakthrough Delta variant infection cases were 251 times higher than those of cases infected with old strains detected between March-April 2020”. |
| 8) Brown et al. | In Barnstable, Massachusetts, Brown et al. found that among 469 cases of COVID-19, 74% were fully vaccinated, and that “the vaccinated had on average more virus in their nose than the unvaccinated who were infected.” |
| 9) Hetemäli et al. | Reporting on a nosocomial hospital outbreak in Finland, Hetemäli et al. observed that “both symptomatic and asymptomatic infections were found among vaccinated health care workers, and secondary transmission occurred from those with symptomatic infections despite use of personal protective equipment.” |
| 10) Shitrit et al. | In a hospital outbreak investigation in Israel, Shitrit et al. observed “high transmissibility of the SARS-CoV-2 Delta variant among twice vaccinated and masked individuals.” They added that “this suggests some waning of immunity, albeit still providing protection for individuals without comorbidities.” |
| 11) UK COVID-19 vaccine Surveillance Report for week #42 | In the UK COVID-19 vaccine Surveillance Report for week #42, it was noted that there is “waning of the N antibody response over time” and “that N antibody levels appear to be lower in individuals who acquire infection following 2 doses of vaccination.” The same report (Table 2, page 13), shows the in the older age groups above 30, the double vaccinated persons have greater infection risk than the unvaccinated, presumably because the latter group include more people with stronger natural immunity from prior Covid disease. As a contrast, the vaccinated people had a lower risk of death than the unvaccinated, across all age groups, indicating that vaccines provide more protection against death than against infection. See also UK PHE reports 43, 44, 45, 46 for similar data. |
| 12) Levin et al. | In Israel, Levin et al. “conducted a 6-month longitudinal prospective study involving vaccinated health care workers who were tested monthly for the presence of anti-spike IgG and neutralizing antibodies”. They found that “six months after receipt of the second dose of the BNT162b2 vaccine, humoral response was substantially decreased, especially among men, among persons 65 years of age or older, and among persons with immunosuppression.” |
| 13) Rosenberg et al. | In a study from New York State, Rosenberg et al. reported that “During May 3–July 25, 2021, the overall age-adjusted vaccine effectiveness against hospitalization in New York was relatively stable 89.5%–95.1%). The overall age-adjusted vaccine effectiveness against infection for all New York adults declined from 91.8% to 75.0%.” |
| 14) Suthar et al. | Suthar et al. noted that “Our data demonstrate a substantial waning of antibody responses and T cell immunity to SARS-CoV-2 and its variants, at 6 months following the second immunization with the BNT162b2 vaccine.” |
| 15) Nordström et al. | In a study from Umeå University in Sweden, Nordström et al. observed that “vaccine effectiveness of BNT162b2 against infection waned progressively from 92% (95% CI, 92-93, P<0·001) at day 15-30 to 47% (95% CI, 39-55, P<0·001) at day 121-180, and from day 211 and onwards no effectiveness could be detected (23%; 95% CI, -2-41, P=0·07).” |
| 16) Yahi et al. | Yahi et al. have reported that “in the case of the Delta variant, neutralizing antibodies have a decreased affinity for the spike protein, whereas facilitating antibodies display a strikingly increased affinity. Thus, antibody dependent enhancement may be a concern for people receiving vaccines based on the original Wuhan strain spike sequence.” |
| 17) Goldberg et al. | (BNT162b2 Vaccine in Israel) reported that “immunity against the delta variant of SARS-CoV-2 waned in all age groups a few months after receipt of the second dose of vaccine.” |
| 18) Singanayagam et al. | examined the transmission and viral load kinetics in vaccinated and unvaccinated individuals with mild delta variant infection in the community. They found that (in 602 community contacts (identified via the UK contract-tracing system) of 471 UK COVID-19 index cases were recruited to the Assessment of Transmission and Contagiousness of COVID-19 in Contacts cohort study and contributed 8145 upper respiratory tract samples from daily sampling for up to 20 days) “vaccination reduces the risk of delta variant infection and accelerates viral clearance. Nonetheless, fully vaccinated individuals with breakthrough infections have peak viral load similar to unvaccinated cases and can efficiently transmit infection in household settings, including to fully vaccinated contacts.” |
| 19) Keehner et al. | in NEJM, has recently reported on the resurgence of SARS-CoV-2 infection in a highly vaccinated health system workforce. Vaccination with mRNA vaccines began in mid-December 2020; by March, 76% of the workforce had been fully vaccinated, and by July, the percentage had risen to 87%. Infections had decreased dramatically by early February 2021…”coincident with the end of California’s mask mandate on June 15 and the rapid dominance of the B.1.617.2 (delta) variant that first emerged in mid-April and accounted for over 95% of UCSDH isolates by the end of July, infections increased rapidly, including cases among fully vaccinated persons…researchers reported that the “dramatic change in vaccine effectiveness from June to July is likely to be due to both the emergence of the delta variant and waning immunity over time.” |
| 20) Juthani et al. | Juthani et al. sought to describe the impact of vaccination on admission to hospital in patients with confirmed SARS-CoV-2 infection using real-world data collected by the Yale New Haven Health System. “Patients were considered fully vaccinated if the final dose (either second dose of BNT162b2 or mRNA-1273, or first dose of Ad.26.COV2.S) was administered at least 14 days before symptom onset or a positive PCR test for SARS-CoV-2. In total, we identified 969 patients who were admitted to a Yale New Haven Health System hospital with a confirmed positive PCR test for SARS-CoV-2”…Researchers reported “a higher number of patients with severe or critical illness in those who received the BNT162b2 vaccine than in those who received mRNA-1273 or Ad.26.COV2.S…” |
| 21) the CDC | A very recent study published by the CDC reported that a majority (53%) of patients who were hospitalized with Covid-19-like illnesses were already fully vaccinated with two-dose RNA shots. Table 1 reveals that among the 20,101 immunocompromised adults hospitalized with Covid-19, 10,564 (53%) were fully-vaccinated with the Pfizer or Moderna vaccine (Vaccination was defined as having received exactly 2 doses of an mRNA-based COVID-19 vaccine ≥14 days before the hospitalization index date, which was the date of respiratory specimen collection associated with the most recent positive or negative SARS-CoV-2 test result before the hospitalization or the hospitalization date if testing only occurred after the admission). This highlights the ongoing challenges faced with Delta breakthrough when vaccinated. |
| 22) Eyre, 2021 The impact of SARS-CoV-2 vaccination on Alpha & Delta variant transmission. | Eyre, 2021 looked at The impact of SARS-CoV-2 vaccination on Alpha & Delta variant transmission. They reported that “while vaccination still lowers the risk of infection, similar viral loads in vaccinated and unvaccinated individuals infected with Delta question how much vaccination prevents onward transmission… transmission reductions declined over time since second vaccination, for Delta reaching similar levels to unvaccinated individuals by 12 weeks for ChAdOx1 and attenuating substantially for BNT162b2. Protection from vaccination in contacts also declined in the 3 months after second vaccination…vaccination reduces transmission of Delta, but by less than the Alpha variant.” |
| 23) Levine-Tiefenbrun | Levine-Tiefenbrun, 2021 looked at Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2, and reported the viral load reduction effectiveness declines with time after vaccination, “significantly decreasing at 3 months after vaccination and effectively vanishing after about 6 months.” |
| 24) Puranik, 2021 Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence | Puranik, 2021 looked at a Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence, reporting “In July, vaccine effectiveness against hospitalization has remained high (mRNA-1273: 81%, 95% CI: 33–96.3%; BNT162b2: 75%, 95% CI: 24–93.9%), but effectiveness against infection was lower for both vaccines (mRNA-1273: 76%, 95% CI: 58–87%; BNT162b2: 42%, 95% CI: 13–62%), with a more pronounced reduction for BNT162b2.” |
| 25) Saade, 2021 Live virus neutralization testing in convalescent patients and subjects vaccinated against 19A, 20B, 20I/501Y.V1 and 20H/501Y.V2 isolates of SARS-CoV-2 | Saade, 2021 looked at Live virus neutralization testing in convalescent patients and subjects vaccinated against 19A, 20B, 20I/501Y.V1 and 20H/501Y.V2 isolates of SARS-CoV-2, and reported as “Assessed the neutralizing capacity of antibodies to prevent cell infection, using a live virus neutralization test with different strains [19A (initial one), 20B (B.1.1.241 lineage), 20I/501Y.V1 (B.1.1.7 lineage), and 20H/501Y.V2 (B.1.351 lineage)] in serum samples collected from different populations: two-dose vaccinated COVID-19-naive healthcare workers (HCWs; Pfizer-BioNTech BNT161b2), 6-months post mild COVID-19 HCWs, and critical COVID-19 patients… finding of the present study is the reduced neutralizing response observed towards the 20H/501Y.V2 variant in fully immunized subjects with the BNT162b2 vaccine by comparison to the wild type and 20I/501Y.V1 variant.” |
| 26) Canaday, 2021 Significant reduction in humoral immunity among healthcare workers and nursing home residents 6 months after COVID-19 BNT162b2 mRNA vaccination | Canaday, 2021 looked at Significant reduction in humoral immunity among healthcare workers and nursing home residents 6 months after COVID-19 BNT162b2 mRNA vaccination, reporting “Anti-spike, anti-RBD and neutralization levels dropped more than 84% over 6 months’ time in all groups irrespective of prior SARS-CoV-2 infection. At 6 months post-vaccine, 70% of the infection-naive NH residents had neutralization titers at or below the lower limit of detection compared to 16% at 2 weeks after full vaccination. These data demonstrate a significant reduction in levels of antibody in all groups. In particular, those infection-naive NH residents had lower initial post-vaccination humoral immunity immediately and exhibited the greatest declines 6 months later.” |
| 27) Israel, 2021 Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection | Israel, 2021 looked at Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection, and reported as “To determine the kinetics of SARS-CoV-2 IgG antibodies following administration of two doses of BNT162b2 vaccine, or SARS-CoV-2 infection in unvaccinated individuals…In vaccinated subjects, antibody titers decreased by up to 40% each subsequent month while in convalescents they decreased by less than 5% per month. Six months after BNT162b2 vaccination 16.1% subjects had antibody levels below the sero-positivity threshold of <50 AU/mL, while only 10.8% of convalescent patients were below <50 AU/mL threshold after 9 months from SARS-CoV-2 infection.” |
| 28) Eyran, 2020 The longitudinal kinetics of antibodies in COVID-19 recovered patients over 14 months | Eyran, 2020 examined The longitudinal kinetics of antibodies in COVID-19 recovered patients over 14 months, and found “a significantly faster decay in naïve vaccinees compared to recovered patients suggesting that the serological memory following natural infection is more robust compared to vaccination. Our data highlights the differences between serological memory induced by natural infection vs. vaccination.” |
| 29) Salvatore et al. | Salvatore et al. examined the transmission potential of vaccinated and unvaccinated persons infected with the SARS-CoV-2 Delta variant in a federal prison, July-August 2021. They found a total of 978 specimens were provided by 95 participants, “of whom 78 (82%) were fully vaccinated and 17 (18%) were not fully vaccinated….clinicians and public health practitioners should consider vaccinated persons who become infected with SARS-CoV-2 to be no less infectious than unvaccinated persons.” |
| 30) Andeweg et al. | Andeweg et al. analyzed 28,578 sequenced SARS-CoV-2 samples from individuals with known immune status obtained through national community testing in the Netherlands from March to August 2021. They found evidence for an “increased risk of infection by the Beta (B.1.351), Gamma (P.1), or Delta (B.1.617.2) variants compared to the Alpha (B.1.1.7) variant after vaccination. No clear differences were found between vaccines. However, the effect was larger in the first 14-59 days after complete vaccination compared to 60 days and longer. In contrast to vaccine-induced immunity, no increased risk for reinfection with Beta, Gamma or Delta variants relative to Alpha variant was found in individuals with infection-induced immunity.” |
| 31) Di Fusco et al. | Di Fusco et al. conducted an evaluation of COVID-19 vaccine breakthrough infections among immunocompromised patients fully vaccinated with BNT162b2. “COVID-19 vaccine breakthrough infections were examined in fully vaccinated (≥14 days after 2nd dose) IC individuals (IC cohort), 12 mutually exclusive IC condition groups, and a non-IC cohort.” They found that“of 1,277,747 individuals ≥16 years of age who received 2 BNT162b2 doses, 225,796 (17.7%) were identified as IC (median age: 58 years; 56.3% female). The most prevalent IC conditions were solid malignancy (32.0%), kidney disease (19.5%), and rheumatologic/inflammatory conditions (16.7%). Among the fully vaccinated IC and non-IC cohorts, a total of 978 breakthrough infections were observed during the study period; 124 (12.7%) resulted in hospitalization and 2 (0.2%) were inpatient deaths. IC individuals accounted for 38.2% (N = 374) of all breakthrough infections, 59.7% (N = 74) of all hospitalizations, and 100% (N = 2) of inpatient deaths. The proportion with breakthrough infections was 3 times higher in the IC cohort compared to the non-IC cohort (N = 374 [0.18%] vs. N = 604 [0.06%]; unadjusted incidence rates were 0.89 and 0.34 per 100 person-years, respectively.” |
| 32) Mallapaty (NATURE) | (NATURE) reported that the protective effect of being vaccinated if you already had infection is “relatively small, and dwindles alarmingly at three months after the receipt of the second shot.” Mallapaty further adds what we have been warning the public health community which is that persons infected with Delta have about the same levels of viral genetic materials in their noses “regardless of whether they’d previously been vaccinated, suggesting that vaccinated and unvaccinated people might be equally infectious.” Mallapaty reported on testing data from 139,164 close contacts of 95,716 people infected with SARS-CoV-2 between January and August 2021 in the United Kingdom, and at a time when the Alpha and Delta variants were competing for dominance. The finding was that “although the vaccines did offer some protection against infection and onward transmission, Delta dampened that effect. A person who was fully vaccinated and then had a ‘breakthrough’ Delta infection was almost twice as likely to pass on the virus as someone who was infected with Alpha. And that was on top of the higher risk of having a breakthrough infection caused by Delta than one caused by Alpha.” |
| 33) Chia et al. | Chia et al. reported that PCR cycle threshold (Ct) values were “similar between both vaccinated and unvaccinated groups at diagnosis, but viral loads decreased faster in vaccinated individuals. Early, robust boosting of anti-spike protein antibodies was observed in vaccinated patients, however, these titers were significantly lower against B.1.617.2 as compared with the wildtype vaccine strain.” |
| 34) Wilhelm et al. | Wilhelm et al. reported on reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies. “in vitro findings using authentic SARS-CoV-2 variants indicate that in contrast to the currently circulating Delta variant, the neutralization efficacy of vaccine-elicited sera against Omicron was severely reduced highlighting T-cell mediated immunity as essential barrier to prevent severe COVID-19.” |
| 35) CDC Report | CDC reported on the details for 43 cases of COVID-19 attributed to the Omicron variant. They found that “34 (79%) occurred in persons who completed the primary series of an FDA-authorized or approved COVID-19 vaccine ≥14 days before symptom onset or receipt of a positive SARS-CoV-2 test result.” |
| 36) Dejnirattisai et al. | Dejnirattisai et al. presented live neutralisation titres against SARS-CoV-2 Omicron variant, and examined it relative to neutralisation against the Victoria, Beta and Delta variants. They reported a significant drop in “neutralisation titres in recipients of both AZD1222 and BNT16b2 primary courses, with evidence of some recipients failing to neutralise at all.” |
| 37) Cele et al. | Cele et al. assessed whether Omicron variant escapes antibody neutralization “elicited by the Pfizer BNT162b2 mRNA vaccine in people who were vaccinated only or vaccinated and previously infected.” They reported that Omicron variant “still required the ACE2 receptor to infect but had extensive escape of Pfizer elicited neutralization.” |
| 38) Holm Hansen et al. | Holm Hansen et al.’s Denmark study looked at vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series. A key finding was reported as “VE against Omicron was 55.2% initially following primary BNT162b2 vaccination, but waned quickly thereafter. Although estimated with less precision, VE against Omicron after primary mRNA-1273 vaccination similarly indicated a rapid decline in protection. By comparison, both vaccines showed higher, longer-lasting protection against Delta.” In other words, the vaccine that has failed against Delta is even far worse for Omicron. The table and figure below paint a devastating picture. See where the green dot is (Omicron variant) in the vertical lines (blue is Delta) and the 2 edges of the bars (upper and lower lips) 91 days out for Omicron (3 months). Both Pfizer and Moderna show negative efficacy for Omicron at 31 days (both are below the ‘line of no effect’ or ‘0’). The comparative table is even more devastating for it shows how much less vaccine effectiveness there is for Omicron. For example, at 1-30 days, Pfizer showed 55.2% effectiveness for Omicron versus 86.7% for Delta, and for the same period, Moderna showed 36.7% effectiveness for Omicron versus 88.2% for Delta. |
| 39) UK Health Security Agency | UK reporting showed that boosters protect against symptomatic COVID-19 caused by Omicron for about 10 weeks; the UK Health Security Agency reported protection against symptomatic COVID-19 caused by the variant dropped from 70% to 45% following a Pfizer booster for those initially vaccinated with the shot developed by Pfizer with BioNTech. Specifically reporting by the UK Health Security Agency showed “Among those who received an AstraZeneca primary course, vaccine effectiveness was around 60% 2 to 4 weeks after either a Pfizer or Moderna booster, then dropped to 35% with a Pfizer booster and 45% with a Moderna booster by 10 weeks after the booster. Among those who received a Pfizer primary course, vaccine effectiveness was around 70% after a Pfizer booster, dropping to 45% after 10-plus weeks and stayed around 70 to 75% after a Moderna booster up to 9 weeks after booster.” |
| 40) Buchan et al. | Buchan et al. used a test-negative design to assess vaccine effectiveness against OMICRON or DELTA variants (regardless of symptoms or severity) during November 22 and December 19, 2021. They included persons who had received at least 2 COVID-19 vaccine doses (with at least 1 mRNA vaccine dose for the primary series) and applied multivariable logistic regression modelling analysis to “estimate the effectiveness of two or three doses by time since the latest dose.” They included 3,442 Omicron-positive cases, 9,201 Delta-positive cases, and 471,545 test-negative controls. Following 2 doses, “vaccine effectiveness against Delta infection declined steadily over time but recovered to 93% (95%CI, 92-94%) ≥7 days after receiving an mRNA vaccine for the third dose. In contrast, receipt of 2 doses of COVID-19 vaccines was not protective against Omicron. Vaccine effectiveness against Omicron was 37% (95%CI, 19-50%) ≥7 days after receiving an mRNA vaccine for the third dose.” |
| 41) Public Health Scotland COVID-19 & Winter Statistical Report | Public Health Scotland COVID-19 & Winter Statistical Report ( Publication date: 19 January 2022) provided startling data on page 38 (case rates), page 44 (hospitalization), and page 50 (deaths), showing that the vaccination has failed Delta but critically, is failing omicron. The 2nd inoculation data is of particular concern. Table 14 age-standardized case data is very troubling for it shows across the multiple weeks of study that across each dose (1 vs 2 vs 3 booster inoculations) that the vaccinated are greatly more infected than the unvaccinated, with the 2nd dose being alarmingly elevated (see grey rows). Age-standardized rates of acute hospital admissions are stunningly elevated after 2nd inoculation (over the unvaccinated) during January 2022. Looking at table 16 that reports on the number of confirmed COVID-19 related deaths by vaccination status, we again observe massive elevation in death at the 2ndinoculation. This data indicates to us that the vaccine is associated with infection and is not optimally working against omicron and that the protection is limited, waning rapidly. |
| 42) The UK’s COVID-19 vaccine surveillance report Week 3, 20 January 2022 | The UK’s COVID-19 vaccine surveillance report Week 3, 20 January 2022, raises very serious concern as to the failure of the vaccines on Delta (which is basically now being replaced by omicron for dominance) and omicron. When we look at table 9, page 34 (COVID-19 cases by vaccination status between week 51 2021 and week 2 2022), we see greater case numbers for the 2nd and 3rd inoculations. The important table on page 38, Figure 12 (unadjusted rates of COVID-19 infection, hospitalization and death in vaccinated and unvaccinated populations) shows us a continual pattern in the UK data over the last 2 to 3 to 4 months, with the present reporting showing that persons in receipt of the 3rd inoculation (booster) at far greater risk of infection/cases than the unvaccinated (30 years of age and above age strata). |
| 43) UK Public Health surveillance reports | In the recent UK Public Health surveillance reports Week 9, Week 8, as well as week 7 (UK COVID-19 vaccine surveillance report Week 7 17 February 2022), week 6 (COVID-19 vaccine surveillance report Week 6 10 February 2022) and week 5 for 2022 (COVID-19 vaccine surveillance report Week 5 3 February 2022) as well as the reports accumulated for 2021 since vaccine roll-out, we see that the vaccinated are at higher risk of infection and especially for age groups above 18 years old, as well as hospitalization and even death. This is particularly marked for those in receipt of double vaccinations. There is increased risk of death for those who are triple vaccinated and especially as age increases. The same pattern emerges in the Scottish data. |
| 44.) Regev-Yochay et al. | Regev-Yochay et al. in Israel looked at (publication date March 16th 2022) the immunogenicity and safety of a fourth dose (4th) of either BNT162b2 (Pfizer–BioNTech) or mRNA-1273 (Moderna) administered 4 months after the third dose in a series of three BNT162b2 doses). This was an open-label, nonrandomized clinical study assessing the 4th dose in terms of need beyond the 3rd dose. Among the ‘1050 eligible health care workers enrolled in the Sheba HCW COVID-19 Cohort, 154 received the fourth dose of BNT162b2 and, 1 week later, 120 received mRNA-1273. For each participant, two age-matched controls were selected from the remaining eligible participants’. Researchers further reported that ‘overall, 25.0% of the participants in the control group were infected with the omicron variant, as compared with 18.3% of the participants in the BNT162b2 group and 20.7% of those in the mRNA-1273 group. Vaccine efficacy against any SARS-CoV-2 infection was 30% (95% confidence interval [CI], −9 to 55) for BNT162b2 and 11% (95% CI, −43 to 44) for mRNA-1273…most of the infected participants were potentially infectious, with relatively high viral loads (nucleocapsid gene cycle threshold, ≤25)’. Results suggest that maximal immunogenicity of mRNA vaccines is achieved after three doses. More specifically, researchers ‘observed low vaccine efficacy against infections in health care workers, as well as relatively high viral loads suggesting that those who were infected were infectious. Thus, a fourth vaccination of healthy young health care workers may have only marginal benefits’. |
| 45.) Andrews et al. | Andrews et al. used a test-negative case-control design to estimate vaccine effectiveness against symptomatic disease caused by the omicron and delta (B.1.617.2) variants in England. “Vaccine effectiveness was calculated after primary immunization with two doses of BNT162b2 (Pfizer-BioNTech), ChAdOx1 nCoV-19 (AstraZeneca), or mRNA-1273 (Moderna) vaccine and after a booster dose of BNT162b2, ChAdOx1 nCoV-19, or mRNA-1273.” The results showed that immunization with two doses of ChAdOx1 nCoV-19 or BNT162b2 vaccine gave very limited protection against symptomatic disease caused by the omicron variant. “A BNT162b2 or mRNA-1273 booster after either the ChAdOx1 nCoV-19 or BNT162b2 primary course substantially increased protection, but that protection waned over time.” |
| 46) Hoffmann et al. | Hoffmann et al. published in journal CELL that the OMICRON spike protein (antigen) eluded neutralization by antibodies from “convalescent patients or individuals vaccinated with the BioNTech-Pfizer vaccine (BNT162b2) with 12- to 44-fold higher efficiency than the spike of the Delta variant. Neutralization of the Omicron spike by antibodies induced upon heterologous ChAdOx1 (Astra Zeneca-Oxford)/BNT162b2 vaccination or vaccination with three doses of BNT162b2 was more efficient, but the Omicron spike still evaded neutralization more efficiently than the Delta spike.” Overall, the results showed that the majority of therapeutic antibodies will be ineffective against the Omicron variant and alarmingly, that double inoculation with BNT162b2 (Pfizer) might not “adequately protect against severe disease induced by this variant.” |
| 47) Bar-on et al. | Bar-on et al. published in NEJM under the title: Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. They assessed the Israeli Ministry of Health database and culled data on 1,252,331 persons who were 60 years of age or older and eligible for the fourth dose during a period in which the B.1.1.529 (omicron) variant of SARS-CoV-2 was predominant (January 10 through March 2, 2022). The analysis focused on the rate of confirmed infection and severe Covid-19 as a function of time beginning at 8 days post receipt of a fourth dose (four-dose groups) as compared with that among persons who had received only three doses (three-dose group) and among persons who had received a fourth dose 3 to 7 days earlier (internal control group). They employed a quasi-Poisson regression modelling and with adjustment for confounders, reportedly adjusted for age, sex, demographic group, and calendar day. The key findings underscoring the failure of the 4th dose, is as follows: “Comparing the rate ratio over time since the fourth dose (Figure 2) suggests that the protection against confirmed infection with the omicron variant reaches a maximum in the fourth week after vaccination, after which the rate ratio decreases to approximately 1.1 by the eighth week; these findings suggest that protection against confirmed infection wanes quickly…The adjusted rate of infection in the eighth week after the fourth dose was very similar to those in the control groups; the rate ratio for the three-dose group as compared with the four-dose group was 1.1 (95% CI, 1.0 to 1.2), and the rate ratio for the internal control group as compared with the four-dose group was only 1.0 (95% CI, 0.9 to 1.1).” These findings indicate no difference. We also have concerns with the methodology as it is clear they could not or did not control for pressing confounding (distorting) variables that could impact the findings. This could lead often to overestimation (or underestimation) of treatment effect. For example, did they control for prior infection, did they control for early treatment drug use, did they adjust for behavioral differences in the 4th dose group, or pre-existing conditions, or differential treatment etc. The researchers did account for some biases e.g. “These potential biases include the “healthy vaccinee” bias, in which people who feel ill tend not to get vaccinated in the following days, which leads to a lower number of confirmed infections and severe disease in the four-dose group during the first days after vaccination. Moreover, one would expect that detection bias due to behavioral changes, such as the tendency to perform fewer tests after vaccination, is more pronounced shortly after receipt of the dose.” |
| 48) Durability of BNT162b2 vaccine against hospital and emergency department admissions due to the omicron and delta variants in a large health system in the USA: a test-negative case–control study, Tartof, 2022. | Researchers evaluated the effectiveness and durability of two and three doses of the BNT162b2 (Pfizer–BioNTech) mRNA vaccine against hospital and emergency department admissions due to the delta (B.1.617.2) and omicron variants; a case–control study with a test-negative design, analyzing electronic health records of members of Kaiser Permanente Southern California (KPSC), a large integrated health system in California, USA, from Dec 1, 2021, to Feb 6, 2022; “analyses were done for 11 123 hospital or emergency department admissions. In adjusted analyses, effectiveness of two doses of the BNT162b2 vaccine against the omicron variant was 41% (95% CI 21–55) against hospital admission and 31% (16–43) against emergency department admission at 9 months or longer after the second dose”; researchers also reported that “3 months after receipt of a third dose, waning was apparent against SARS-CoV-2 outcomes due to the omicron variant, including hospital admission.” |
| 49) Laith J. Abu-Raddad et al. (May, 2022): | “Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar”; as we see, the vaccine has failed, VE is <50% (the needed threshold) and ‘0’ deaths; “Two matched retrospective cohort studies to assess the effectiveness of booster vaccination, as compared with that of a two-dose primary series alone, against symptomatic SARS-CoV-2 infection and Covid-19–related hospitalization and death during a large wave of omicron infections from December 19, 2021, through January 26, 2022. The association of booster status with infection was estimated with the use of Cox proportional-hazards regression models.” As we see, the vaccine has failed, VE is <50% (the needed threshold) and ‘0’ deaths. The key findings are as follows to show that the vaccines do not hit the 50% threshold for effectiveness: Effectiveness of BNT162b2 Booster against Omicron Variant “The estimated effectiveness of the BNT162b2 booster (Pfizer) against symptomatic omicron infection, as compared with that of the two-dose primary series, was 49.4% (95% CI, 47.1 to 51.6).” Effectiveness of mRNA-1273 Booster against Omicron Variant “The estimated effectiveness of the mRNA-1273 booster (Moderna), as compared with that of the two-dose primary series, was 47.3% (95% CI, 40.7 to 53.3).” Additional Analyses “For the BNT162b2 vaccine analysis, with the start of follow-up on the 15th day after the booster vaccination, the estimated effectiveness of the booster against symptomatic omicron infection, as compared with that of the two-dose primary series, was 49.9% (95% CI, 47.6 to 52.2) (Fig. S2 and Table S4). The corresponding estimated effectiveness of the mRNA-1273 vaccine was 52.0% (95% CI, 45.1 to 57.9). Both effectiveness estimates were similar to those in the main analysis. The estimated effectiveness of the BNT162b2 vaccine booster against symptomatic omicron infection, as compared with that of the two-dose primary series, was 38.0% (95% CI, 28.8 to 46.0) in persons who received the booster 8 months or less after the second dose and 50.5% (95% CI, 48.2 to 52.8) in those who received it more than 8 months after the second dose. The corresponding estimates of the effectiveness of mRNA-1273 vaccine were 41.5% (95% CI, 32.3 to 49.5) and 56.8% (95% CI, 47.0 to 64.8).” |
| 50) Fleming-Dutra et al. | Fleming-Dutra et al. examined the association of Prior BNT162b2 COVID-19 Vaccination With Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance. They used a test-negative, case-control study conducted from December 2021 to February 2022 during Omicron variant predominance that included 121 952 tests from sites across the US, estimated vaccine effectiveness against symptomatic infection for children 5 to 11 years of age was 60.1% 2 to 4 weeks after dose 2 and 28.9% during month 2 after dose 2. Among adolescents 12 to 15 years of age, estimated vaccine effectiveness was 59.5% 2 to 4 weeks after dose 2 and 16.6% during month 2 (see Figure 2). They concluded that “among children and adolescents, estimated vaccine effectiveness for 2 doses of BNT162b2 against symptomatic infection decreased rapidly”. We see VE dropping below 0 at approximately 4.5 months. |
| 51) Lassaunière et al: | Lassaunière et al: “Neutralizing Antibodies Against the SARS-CoV-2 Omicron Variant (BA.1) 1 to 18 Weeks After the Second and Third Doses of the BNT162b2 mRNA Vaccine”; “Our study found a rapid decline in Omicron-specific serum neutralizing antibody titers only a few weeks after the second and third doses of BNT162b2….the observed decrease in population neutralizing antibody titers corresponds to the decrease in vaccine efficacy against polymerase chain reaction–confirmed Omicron infection in Denmark and symptomatic Omicron infection in the United Kingdom…Taken together, vaccine-induced protective antibody responses following a second and third dose of BNT162b2 are transient and additional booster doses may be necessary, particularly in older people; however, conserved T-cell immunity and non-neutralizing antibodies may still provide protection against hospitalization and death.” |
| 52) Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2 | “The number of cases of SARS-CoV-2 infection per 100,000 person-days at risk (adjusted rate) increased with the time that had elapsed since vaccination with BNT162b2 or since previous infection. Among unvaccinated persons who had recovered from infection, this rate increased from 10.5 among those who had been infected 4 to less than 6 months previously to 30.2 among those who had been infected 1 year or more previously. Among persons who had received a single dose of vaccine after previous infection, the adjusted rate was low (3.7) among those who had been vaccinated less than 2 months previously but increased to 11.6 among those who had been vaccinated at least 6 months previously. Among previously uninfected persons who had received two doses of vaccine, the adjusted rate increased from 21.1 among those who had been vaccinated less than 2 months previously to 88.9 among those who had been vaccinated at least 6 months previously. Among persons who had been previously infected with SARS-CoV-2 (regardless of whether they had received any dose of vaccine or whether they had received one dose before or after infection), protection against reinfection decreased as the time increased since the last immunity-conferring event; however, this protection was higher than that conferred after the same time had elapsed since receipt of a second dose of vaccine among previously uninfected persons. A single dose of vaccine after infection reinforced protection against reinfection.” |
| 53) CDC and waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance — VISION Network, 10 States, August 2021–January 2022, Ferdinands, 2022: | “During the Omicron-predominant period, VE against COVID-19–associated ED/UC encounters was lower overall compared with that during the Delta-predominant period and waned after the second dose, from 69% within 2 months of vaccination to 37% at ≥5 months after vaccination (p<0.001). Protection increased after a third dose, with VE of 87% among those vaccinated within the past 2 months; however, VE after 3 doses declined to 66% among those vaccinated 4–5 months earlier and 31% among those vaccinated ≥5 months earlier”…in a multistate analysis of 241,204 ED/UC encounters and 93,408 hospitalizations among adults with COVID-19–like illness during August 26, 2021–January 22, 2022, estimates of VE against laboratory-confirmed COVID-19 were lower during the Omicron-predominant than during the Delta-predominant period, after accounting for both number of vaccine doses received and time since vaccination. During both periods, VE after receipt of a third dose was always higher than VE following a second dose; however, VE waned with increasing time since vaccination.” |
| 54) Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales, Agrawal et al., October, 2022 | “There was an increased risk of severe COVID-19 outcomes 10 weeks after completing the primary doses of BNT162b2 or ChAdOx1 nCoV-19 (≥20 weeks vs 3–9 weeks; aRR 4·55 [95% CI 4·16–4·99]). Individuals with a greater number of comorbidities (≥5 comorbidities vs none; 7·98 [7·73–8·24], who were older (aged ≥80 years vs 18–49 years; 8·12 [7·89–8·35]), who had a higher BMI (≥40 vs 18·5–24·9; 1·75 [1·69–1·82]), or who were male (male vs female; 1·19 [1·17–1·21]) were also associated with increased risk of severe COVID-19 outcomes.” This UK-wide population-based investigation of over 16 million in England, Northern Ireland, Scotland, and Wales has found that, after the first vaccine booster, older people, those with high multimorbidity, and those with certain underlying health conditions remain at highest risk of COVID-19-related hospitalization and death. These findings are very problematic for the vaccine advocates. The COVID gene injection vaccine has failed, is non-sterilizing, non-neutralizing, does not protect the upper airways (does not prevent infection or transmission) and does not effectively or properly protect the lower lungs from severe disease. |
| 55) Waning of first- and second-dose ChAdOx1 and BNT162b2 COVID-19 vaccinations: a pooled target trial study of 12.9 million individuals in England, Northern Ireland, Scotland and Wales, Kerr, 2022 | “For Doses 1 and 2 of ChAdOx1 and Dose 1 of BNT162b2, VE/rVE reached zero by approximately Days 60-80 and then went negative. By Day 70, VE/rVE was -25% (95% CI: -80 to 14) and 10% (95% CI: -32 to 39) for Doses 1 and 2 of ChAdOx1, respectively, and 42% (95% CI: 9 to 64) and 53% (95% CI: 26 to 70) for Doses 1 and 2 of BNT162b2, respectively. rVE for Dose 2 of BNT162b2 remained above zero throughout and reached 46% (95% CI: 13 to 67) after 98 days of follow-up. Found strong evidence of waning in VE/rVE for Doses 1 and 2 of ChAdOx1, as well as Dose 1 of BNT162b2.” These finding are not unknown to public health authorities. In fact, CDC Director Rochelle Walensky has said that the Covid vaccines are working “exceptionally well” against severe illness and death, but “what they can’t do anymore is prevent transmission.” What these studies show, are that vaccines are important to reduce severe disease and death, but unable to prevent the disease from spreading and eventually infect most of us. That is, while the vaccines provide individual benefits to the vaccinee, and especially to older high-risk people, the public benefit of universal vaccination is in grave doubt. As such, Covid vaccines should not be expected to contribute to eliminating the communal spread of the virus or the reaching of herd immunity. This unravels the rationale for vaccine mandates and passports. |
| 56.) Six-Month Follow-up after a Fourth BNT162b2 Vaccine Dose, Canetti & Regev-Yochay, 2022 | “Among the participants who had not had previous SARS-CoV-2 infection, 6113 were included in the analysis of humoral response and 11,176 in the analysis of vaccine effectiveness (Fig. S1 and Tables S2 and S3). Antibody response peaked at approximately 4 weeks, waned to levels seen before the fourth dose by 13 weeks, and stabilized thereafter. Throughout the 6-month follow-up period, the adjusted weekly levels of IgG and neutralizing antibodies were similar after receipt of the third and fourth doses and were markedly higher than the levels seen after receipt of the second dose (Figure 1A and 1B and Table S4). The cumulative incidence curve is shown in Figure S2, and vaccine effectiveness is shown in Figure 1C. Receipt of the fourth BNT162b2 vaccine dose conferred more protection against SARS-CoV-2 infection than that afforded by the receipt of three vaccine doses (with receipt of the third dose having occurred at least 4 months earlier) (overall vaccine effectiveness, 41%; 95% confidence interval [CI], 35 to 47). Time-specific vaccine effectiveness (which, in our analysis, compared infection rates among participants who had not yet been infected since vaccination) waned with time, decreasing from 52% (95% CI, 45 to 58) during the first 5 weeks after vaccination to −2% (95% CI, −27 to 17) at 15 to 26 weeks.” 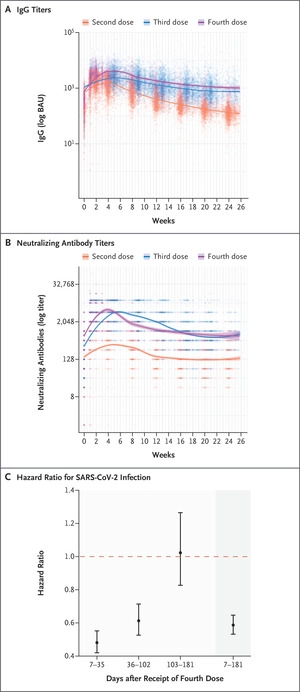  |
| 57) Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants, Tseng, 2022 | “The 2- dose VE against omicron infection at 14-90 days was 44.0% (95% CI, 35.1–51.6%) but declined quickly. The 3-dose VE was 93.7% (92.2–94.9%) and 86.0% (78.1–91.1%) against delta infection and 71.6% (69.7–73.4%) and 47.4% (40.5–53.5%) against omicron infection at 14-60 days and >60 days, respectively. The 3-dose VE was 29.4% (0.3–50.0%) against omicron infection in immunocompromised individuals. The 3-dose VE against hospitalization with delta or omicron was >99%. Our findings demonstrate high, durable 3-dose VE against delta infection but lower effectiveness against omicron infection, particularly among immunocompromised people. However, 3- dose VE was high against hospitalization with delta or omicron.” |
| 58) Rate of SARS-CoV-2 Reinfection During an Omicron Wave in Iceland, Eythorsson, 2022 | “11 536 PCR-positive persons were included. The mean (SD) age was 34 (19) years (median, 31 years; range, 0-102 years), 5888 (51%) were male, 2942 (25.5%) had received at least 1 dose of vaccine, and the mean (SD) time from initial infection was 287 (191) days (median, 227 days; range, 60-642 days); The probability of reinfection increased with time from the initial infection (odds ratio of 18 months vs 3 months, 1.56; 95% CI, 1.18-2.08) (Figure) and was higher among persons who had received 2 or more doses compared with 1 dose or less of vaccine (odds ratio, 1.42; 95% CI, 1.13-1.78)” |
| 59) Effectiveness of mRNA-1273 against infection and COVID-19 hospitalization with SARSCoV-2 Omicron subvariants: BA.1, BA.2, BA.2.12.1, BA.4, and BA.5, Tseng, 2022 | “While 3-dose VE against BA.1 infection was high and waned slowly, VE against BA.2, BA.2.12.1, BA.4, and BA.5 infection was initially moderate to high (61.0%-90.6% 14-30 days post third dose) and waned rapidly. The 4-dose VE against infection with BA.2, BA.2.12.1, and BA.4 ranged between 64.3%-75.7%, and was low (30.8%) against BA.5 14-30 days post fourth dose, disappearing beyond 90 days for all subvariants.” |
| 60) Effectiveness of COVID-19 Vaccines Over 13 Months Covering the Period of the Emergence of the Omicron Variant in the Swedish Population, Yu, 2022 | “Two vaccine doses showed long-lasting good protection against infection before Omicron (VE were above 85% for all time intervals), but less protection against Omicron infection (dropped to 43% by week four and no protection by week 14). Similarly, VE against hospitalization was high and stable before Omicron, but showed clear waning during the Omicron period, although VE estimates were substantially higher (above 80% to week 25, dropping to 40% by week 40) than against infection.” |
| 61) Long-term COVID-19 booster effectiveness by infection history and clinical vulnerability and immune imprinting, Chemaitelly, 2022 | “Booster effectiveness relative to primary series was 41.1% (95% CI: 40.0-42.1%) against infection and 80.5% (95% CI: 55.7-91.4%) against severe, critical, or fatal COVID-19, over one-year follow-up after the booster. Among persons clinically vulnerable to severe COVID-19, effectiveness was 49.7% (95% CI: 47.8-51.6%) against infection and 84.2% (95% CI: 58.8-93.9%) against severe, critical, or fatal COVID-19. Effectiveness against infection was highest at 57.1% (95% CI: 55.9-58.3%) in the first month after the booster but waned thereafter and was modest at only 14.4% (95% CI: 7.3-20.9%) by the sixth month. In the seventh month and thereafter, coincident with BA.4/BA.5 and BA.2.75* subvariant incidence, effectiveness was progressively negative reaching -20.3% (95% CI: -55.0-29.0%) after one year of follow-up. Similar levels and patterns of protection were observed irrespective of prior infection status, clinical vulnerability, or type of vaccine (BNT162b2 versus mRNA-1273).”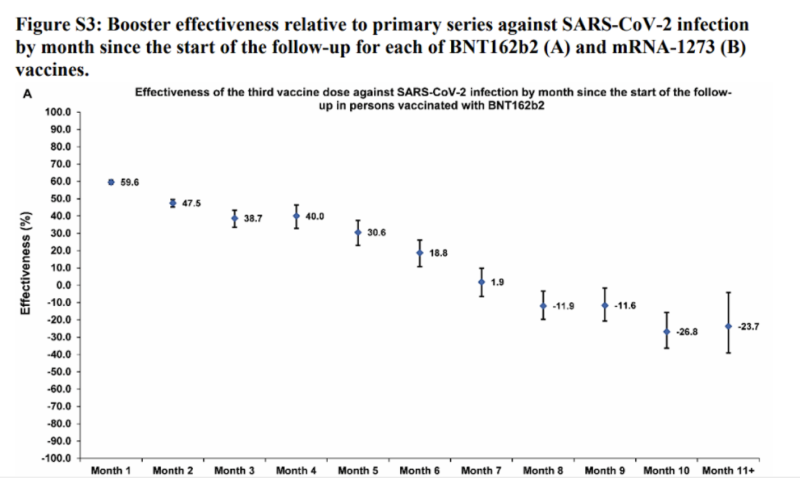  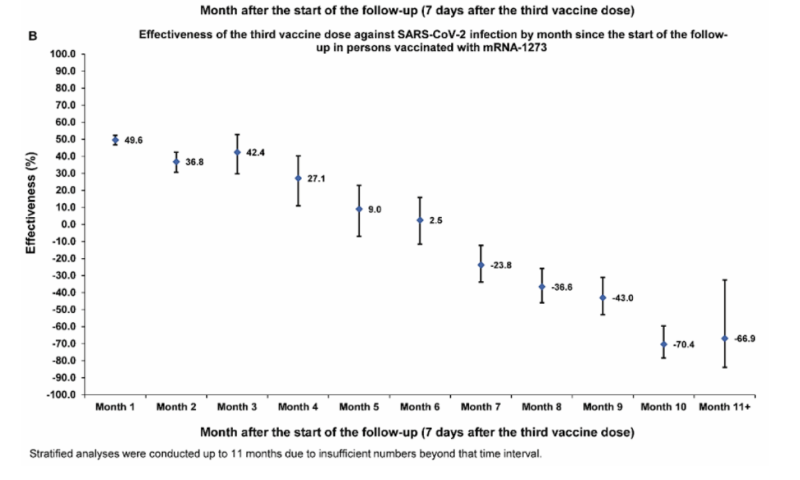  |
| 62) Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants, Wang, 2022 | “BQ.1, BQ.1.1, XBB, and XBB.1 are the most resistant SARS-CoV-2 variants to date; Serum neutralization was markedly reduced, including with the bivalent booster; All clinical monoclonal antibodies were rendered inactive against these variants; The ACE2 affinity of these variants were similar to their parental strains; The BQ and XBB subvariants of SARS-CoV-2 Omicron are now rapidly expanding, possibly due to altered antibody evasion properties deriving from their additional spike mutations. Here, we report that neutralization of BQ.1, BQ.1.1, XBB, and XBB.1 by sera from vaccinees and infected persons was markedly impaired, including sera from individuals boosted with a WA1/BA.5 bivalent mRNA vaccine. Titers against BQ and XBB subvariants were lower by 13-81-fold and 66-155-fold, respectively, far beyond what had been observed to date. Monoclonal antibodies capable of neutralizing the original Omicron variant were largely inactive against these new subvariants, and the responsible individual spike mutations were identified. These subvariants were found to have similar ACE2-binding affinities as their predecessors. Together, our findings indicate that BQ and XBB subvariants present serious threats to current COVID-19 vaccines, render inactive all authorized antibodies, and may have gained dominance in the population because of their advantage in evading antibodies.” |
| 63) Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1, and XBB.1 by parental mRNA vaccine or a BA.5-bivalent booster, Kurhade, 2022 | “The newly emerged SARS-CoV-2 Omicron sublineages, including the BA.2-derived BA.2.75.2 and the BA.5-derived BQ.1.1 and XBB.1, have accumulated additional spike mutations that may affect vaccine effectiveness. Here we report neutralizing activities of three human serum panels collected from individuals 23–94 days after dose 4 of a parental mRNA vaccine, 14–32 days after a BA.5-bivalent-booster from individuals with 2–4 previous doses of parental mRNA vaccine, or 15–32 days after a BA.5-bivalent-booster from individuals with previous SARS-CoV-2 infection and 2–4 doses of parental mRNA vaccine. The results showed that a BA.5-bivalent-booster elicited a high neutralizing titer against BA.4/5 measured at 14- to 32-day post-boost; however, the BA.5-bivalent-booster did not produce robust neutralization against the newly emerged BA.2.75.2, BQ.1.1, or XBB.1. Previous infection significantly enhanced the magnitude and breadth of BA.5-bivalent-booster-elicited neutralization. Our data support a vaccine update strategy that future boosters should match newly emerged circulating SARS-CoV-2 variants.” |
| 64) Effectiveness of the Coronavirus Disease 2019 (COVID-19) Bivalent Vaccine, Shrestha, 2022 | “A retrospective cohort study conducted at the Cleveland Clinic Health System (CCHS) in the United States. Researchers included employees on the very day that the bivalent COVID-19 vaccine was first available. ‘Protection provided by vaccination (analyzed as a time-dependent covariate) was evaluated using Cox proportional hazards regression.’ Findings focused on 51,011 employees of which 20,689 (41%) had a prior documented COVID-19 infection (episode), and whereby 42,064 (83%) received at least two doses of the vaccine. ‘The majority of infections in Ohio were caused by the BA.4 or BA.5 lineages of the Omicron variant during the first 10 weeks of the study, based on SARS-CoV-2 variant monitoring data available from the Ohio Department of Health. By December, the BQ.1, BQ.1.1, and BF.7 lineages accounted for a substantial proportion of the infections.’ ‘By the end of the study, 10804 (21%) were bivalent vaccine boosted. The bivalent vaccine was the Pfizer vaccine in 9595 (89%) and the Moderna vaccine in the remaining 1178. Altogether, 2452 employees (5%) acquired COVID-19 during the 13 weeks of the study.’ ‘The calculated overall vaccine effectiveness from the model was 30% (95% C.I., 20% – 39%)…when the Omicron BA.4/BA.5 lineages were the predominant circulating strains.’ ‘The multivariable analyses also found that, the more recent the last prior COVID-19 episode was the lower the risk of COVID-19, and that the greater the number of vaccine doses previously received the higher the risk of COVID-19.” |
| 65) Effectiveness of second booster compared to first booster and protection conferred by previous SARS CoV-2 infection against symptomatic Omicron BA.2 and BA.4/5 in France, Tamandjou, 2023 | “We included symptomatic ≥60 years old individuals tested for SARSCoV-2 in March 21-October 30, 2022. Compared to a 181-210 days old first booster, a second booster restored protection with an effectiveness of 39% [95%CI: 38% – 41%], 7-30 days postvaccination This gain in protection was lower than the one observed with the first booster, at equal time points since vaccination.” |
| 66) Extended SARS-CoV-2 RBD booster vaccination induces humoral and cellular immune tolerance in mice, Gao, 2023 | i) Our findings demonstrate potential risks with the continuous use of SARS-CoV-2 vaccine boosters, providing immediate implications for the global COVID-19 vaccination enhancement strategies. ii) Whether such re-establishment of vaccine-induced immune response could be repeated by continued application of boosters is being questioned, yet largely unknown at present. Here, we compared the effects of repeated RBD vaccine boosters with a conventional immunization course to those with an extended vaccination strategy, in a Balb/c mice model. iii) We found that the protective effects from the humoral immunity and cellular immunity established by the conventional immunization were both profoundly impaired during the extended vaccination course. Specifically, extended vaccination not only fully impaired the amount and the neutralizing efficacy of serum RBD-specific antibodies, but also shortened the long-term humoral memory. iv) This is associated with immune tolerance in germinal center response, along with decreased numbers of spleen germinal center B and Tfh cells. Moreover, we demonstrated that extended immunization reduced the functional responses of CD4+ and CD8+T cells, restrained the population of memory T cells, and up-regulated the expression of PD-1 and LAG-3 in Te sub-type cells. v) An increased percentile of Treg cells was also observed, accompanied by significant elevation of IL-10 production. Together, we provided crucial evidence that repetitive administration of RBD booster vaccines may negatively impact the immune response established by a conventional vaccination course and promote adaptive immune tolerance.’ vi) Continued vaccination promoted the formation of a prominent adaptive immune tolerance and profoundly impaired the established immune response with the conventional course, evidenced by significant reductions in antigen specific antibody and T cell response, a loss of immune memory and form of immunosuppression micro-environment. |
| 67) Effect of prior infection, vaccination, and hybrid immunity against symptomatic BA.1 and BA.2 Omicron infections and severe COVID-19 in Qatar, Altarawneh, March 2022 | “Qatar researchers investigated SARS-CoV-2 Omicron symptomatic BA.1 infection, symptomatic BA.2 infection, BA.1 hospitalization and death, and BA.2 hospitalization and death, between December 23, 2021 and February 21, 2022. The researchers conducted 6 national, matched, test-negative case-control studies were conducted to examine effectiveness of BNT162b2 (Pfizer-BioNTech) vaccine, mRNA-1273 (Moderna) vaccine, natural immunity due to prior infection with pre-Omicron variants, and hybrid immunity from prior infection and vaccination. They found that “Effectiveness of only prior infection against symptomatic BA.2 infection was 46.1% (95% CI: 39.5-51.9%). Effectiveness of only two-dose BNT162b2 vaccination was negligible at -1.1% (95% CI: -7.1-4.6), but nearly all individuals had received their second dose several months earlier. Effectiveness of only three-dose BNT162b2 vaccination was 52.2% (95% CI: 48.1-55.9%). Effectiveness of hybrid immunity of prior infection and two-dose BNT162b2 vaccination was 55.1% (95% CI: 50.9-58.9%).” The key finding was “There are no discernable differences in the effects of prior infection, vaccination, and hybrid immunity against BA.1 versus BA.2.” |
| 68) Effectiveness of a fourth dose of mRNA COVID-19 vaccine against all-cause mortality in long-term care facility residents and in the oldest old: A nationwide, retrospective cohort study in Sweden, Nordström, 2022 | “From 7 days after baseline and onwards, there were 1119 deaths in the LTCF cohort during a median follow-up of 77 days and a maximum follow-up of 126 days. During days 7 to 60, the VE of the fourth dose was 39% (95% CI, 29-48), which declined to 27% (95% CI, -2-48) during days 61 to 126. In the cohort of all individuals aged ≥80 years, there were 5753 deaths during a median follow-up of 73 days and a maximum follow-up of 143 days. During days 7 to 60, the VE of the fourth dose was 71% (95% CI, 69-72), which declined to 54% (95% CI, 48-60) during days 61 to 143.” |
| 69) Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden, Nordström, 2022 | “For the outcome SARS-CoV-2 infection of any severity, the vaccine effectiveness of BNT162b2 waned progressively over time, from 92% (95% CI 92 to 93; p<0·001) at 15-30 days, to 47% (39 to 55; p<0·001) at 121-180 days, and to 23% (-2 to 41; p=0·07) from day 211 onwards. Waning was slightly slower for mRNA-1273, with a vaccine effectiveness of 96% (94 to 97; p<0·001) at 15-30 days and 59% (18 to 79; p=0·012) from day 181 onwards. Waning was also slightly slower for heterologous ChAdOx1 nCoV-19 plus an mRNA vaccine, for which vaccine effectiveness was 89% (79 to 94; p<0·001) at 15-30 days and 66% (41 to 80; p<0·001) from day 121 onwards. By contrast, vaccine effectiveness for homologous ChAdOx1 nCoV-19 vaccine was 68% (52 to 79; p<0·001) at 15-30 days, with no detectable effectiveness from day 121 onwards (-19% [-98 to 28]; p=0·49). For the outcome of severe COVID-19, vaccine effectiveness waned from 89% (82 to 93; p<0·001) at 15-30 days to 64% (44 to 77; p<0·001) from day 121 onwards. Overall, there was some evidence for lower vaccine effectiveness in men than in women and in older individuals than in younger individuals.” |
| 70) Neutralization against BA.2.75.2, BQ.1.1, and XBB from mRNA Bivalent Booster, Davis-Gardner, 2023 | “Used the FRNT in a VeroE6/TMPRSS2 cell line1 to compare the neutralizing activity in serum samples obtained from participants in three cohorts: the first cohort comprised 12 participants 7 to 28 days after one monovalent booster; the second, 11 participants 6 to 57 days after a second monovalent booster; and the third, 12 participants 16 to 42 days after a bivalent booster. In all three cohorts, neutralization activity was lower against all omicron subvariants than against the WA1/2020 strain; neutralizing activity was lowest against the XBB subvariant (Figure 1 and Fig. S2). In the cohort that received one monovalent booster, the FRNT50 GMTs were 857 against WA1/2020, 60 against BA.1, 50 against BA.5, 23 against BA.2.75.2, 19 against BQ.1.1, and below the limit of detection against XBB. In the cohort that received two monovalent boosters, the FRNT50 GMTs were 2352 against WA1/2020, 408 against BA.1, 250 against BA.5, 98 against BA.2.75.2, 73 against BQ.1.1, and 37 against XBB. The results in both of these cohorts correspond with neutralization titers against BA.1 and BA.5 that were 5 to 9 times as low as that against WA1/2020 and neutralization titers against BA.2.75.2, BQ.1.1, and XBB that were 23 to 63 times as low as that against WA1/2020.” |
| 71) Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5, Hachmann, 2022 | “Six months after the initial two BNT162b2 immunizations, the median neutralizing antibody pseudovirus titer was 124 against WA1/2020 but less than 20 against all the tested omicron subvariants. Two weeks after administration of the booster dose, the median neutralizing antibody titer increased substantially, to 5783 against the WA1/2020 isolate, 900 against the BA.1 subvariant, 829 against the BA.2 subvariant, 410 against the BA.2.12.1 subvariant, and 275 against the BA.4 or BA.5 subvariant. Among the participants with a history of Covid-19, the median neutralizing antibody titer was 11,050 against the WA1/2020 isolate, 1740 against the BA.1 subvariant, 1910 against the BA.2 subvariant, 1150 against the BA.2.12.1 subvariant, and 590 against the BA.4 or BA.5 subvariant.” |
Join the conversation:


Published under a Creative Commons Attribution 4.0 International License
For reprints, please set the canonical link back to the original Brownstone Institute Article and Author.









