You might have heard that the AstraZeneca Covid-19 injectable product has been withdrawn from market use. It’s true. They’ve initiated a worldwide recall of their product as of May 8, 2024.
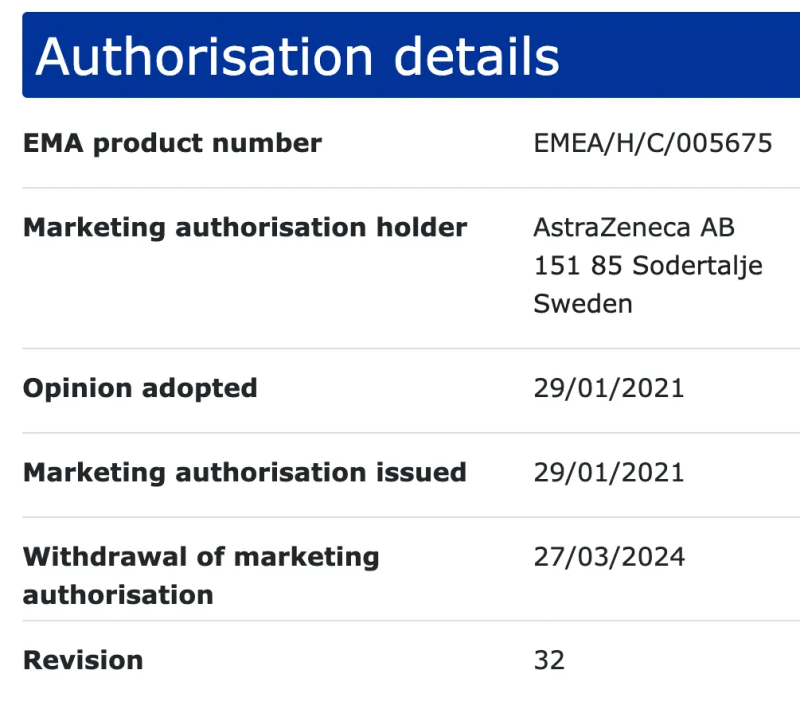

This comes months after the official withdrawal of marketing authorization, but I suppose, better late than never?
According to the CDC, a vaccine/product is withdrawn or recalled rarely, and its recall has always been due to a lack of efficacy or safety of the product in question. The Rotavirus vaccine has been recalled, for example, due to caused intussusception. Gardasil was also recalled, among others.
If you head to CDC website on Vaccine Recalls, you’ll find the following unprompted paragraph under the heading “Why would a vaccine, or certain batches of a vaccine, be withdrawn or recalled?”
Several vaccine lots have been recalled in recent years because of a possible safety concern before anyone reported any injury. Rather, the manufacturer’s quality testing noticed some irregularity in some vaccine vials. In these cases, the safety of these vaccines was monitored continuously before and after they were in use. CDC analyzed reports to the Vaccine Adverse Event Reporting System (VAERS) to search for any side effects that might have been caused by the irregularity, and found none. Any time such an irregularity is found in a vaccine lot which could make it unsafe, the manufacturer, in collaboration with the U.S. Food and Drug Administration (FDA), will recall it immediately.
So all you would need to prevent a recall is to find no [associated] side effects in VAERS. Interesting. Take a look at this graph again.
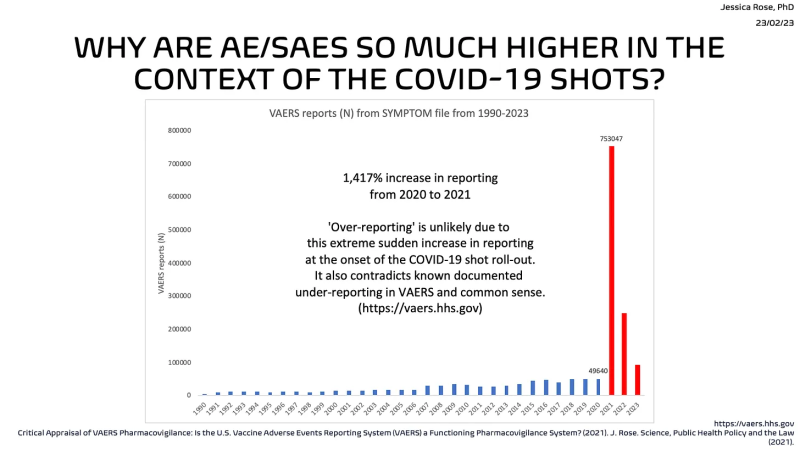

No side effects, eh? What is up with that big red bar then, in 2021? I am waiting to hear, CDC.
Imagine now, after so many years, one of the Covid-19 products (AstraZeneca) has officially been recalled. What this means then, according to the CDC, is that there are admitted side effects caused by the product. We all know about the clotting and Thrombotic Thrombocytopenic Syndrome (TTS). We have all known this for a long time.
What could I possibly be getting at here? I guess what I am getting at is that there has been enough of a death safety signal in VAERS since January 2021 to back a recall of the products unleashed in the United States, namely, the Mod-e-RNA, Pfizer, and Janssen products.
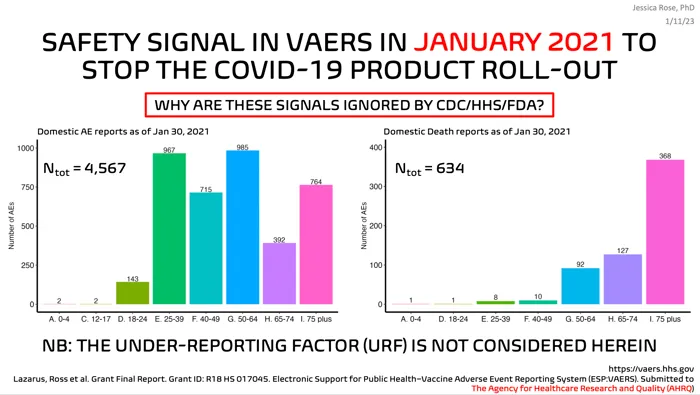

They are ignoring the signals. Willfully. And people are getting hurt and even dying. That’s intent. Because there’s knowledge.
It’s wrong.
Recall them all.
Republished from the author’s Substack
Join the conversation:


Published under a Creative Commons Attribution 4.0 International License
For reprints, please set the canonical link back to the original Brownstone Institute Article and Author.









