The evidence accumulated very early on in the pandemic that the use of sequenced multi-drug therapeutics (SMDT) under physician guidance was beneficial and that some medications were safe and effective. We refer to repurposed therapeutics that have been regulatory approved and have been used in some instances for decades for other illnesses.
We have extensively written and published treatment algorithms and protocols as well as evidence of the benefit of early outpatient (ambulatory) treatment of SARS-CoV-2 virus and the consequent disease COVID-19 (1, 2, 3, 4, 5, 6). With highly targeted and SMDT regimens that include early application of antiviral drugs, combined with corticosteroids and anti-platelet/anti-thrombotic/anti-clotting therapeutics, the risk of hospitalization is significantly reduced by as much as by 85 to 90%, and risk of death is eliminated for high-risk patients and younger individuals presenting with severe symptoms.
COVID-19 presents as either a mild-flu-like condition (asymptomatic or mild symptoms) or more serious illness in those at high risk. A small fraction of persons infected with COVID virus progress to more serious illness (typically elderly with underlying medical conditions, obese or younger with underlying medical conditions/risks factors). The complex and multidimensional pathophysiology of life-threatening COVID-19 illness including viral mediated organ damage, cytokine storm, and thrombosis warrants early interventions to address all components of the illness.
As a brief background, the illness involves three phases 1) an initial viral replication phase whereby the virus hijacks the metabolic machinery of the cells which then begins to synthesize new viral particles ii) a more advanced inflammatory hyper-dysregulated immune-modulatory florid pneumonia phase whereby there is a cytokine storm and problematic gas exchange known as acute respiratory distress syndrome; ARDS. ARDS is generally the cause of most deaths attributed to COVID-19; and iii) a thrombotic blood clotting phase whereby microthrombi develop within the lungs and in the vasculature, leading to disastrous complications including profound hypoxemia, stroke, and heart attacks.
The ideal situation is to arrest the virus in the initial phase when symptoms have just emerged, while the patient is still within the home setting or extended care setting. The goal is to prevent hospitalization and death.
In countries where there is and was a reluctance to treat infected and symptomatic high-risk persons early, this therapeutic nihilism resulted in escalating symptoms, delayed in-hospital care and death. Fortunately, prompt and early initiation of SMDT is a widely and currently available solution to stem the tide of hospitalizations and death.
Viral illnesses such as COVID-19, with complex pathophysiology, do not respond to one drug treatment but require a multi-drug approach. We have to hit the virus with multiple therapeutics. This multipronged therapeutic approach includes 1) adjuvant nutritional supplements; 2) combination intracellular anti-infective therapy (antivirals and antibiotics); 3) inhaled/oral corticosteroids and colchicine; 4) antiplatelet agents/anticoagulants; 5) supportive care including supplemental oxygen, monitoring and telemedicine.
Randomized trials of individual, novel oral therapies have not delivered effective tools. No single therapeutic option thus far has been adequate, but combinations have been employed very successfully in clinical practice. Treating physicians who were courageous and brave felt it was urgent to apply the SMDT approach universally to benefit large numbers of acute COVID-19 patients, reducing their intensity and duration of symptoms and saving them from hospitalization and death. The key is use of early treatment as soon as symptoms develops when the virus is early in the replication phase.
This brief compilation (Table 1 and Figures 1 & 2) describes a cursory summary with the direct url links of therapeutics that have been shown some degree of effectiveness if infected with COVID-19 virus in any of its variant forms including Delta and Omicron.
While the COVID-19 emergency is winding down, with Omicron offering an exit off-ramp, variants, including the Delta and Omicron variant, still exist and will continue to. We therefore felt the public (and particularly those at high-risk) should be aware of known treatment options. While most people and especially young persons and children are indeed at very low risk of illness and especially from the very mild near ‘common-cold’ Omicron variant, this early treatment guidance provides an important resource that can be life saving when needed.
This piece covers:
- Ivermectin
- Doxycycline
- Vitamin D
- Zinc
- Colchicine
- Bromhexine
- Budesonide
- Dexamethasone
- Monoclonal Antibodies
- Quercetin
- Fluvoxamine
- Prednisone
- Azithronmicin
- Hydroxychloroquine
Assisting with this article are
- Dr. Paul E. Alexander, MSc, PhD (PublicHealth.news; TheUNITYProject)
- Dr. Harvey Risch, MD, PhD (Yale School of Public Health)
- Dr. Howard Tenenbaum, PhD ( Faculty of Medicine, University of Toronto)
- Dr. Ramin Oskoui, MD (Foxhall Cardiology, Washington)
- Dr. Peter McCullough, MD (Truth for Health Foundation (TFH)), Texas
- Dr. Parvez Dara, MD (consultant, Medical Hematologist and Oncologist)
- Mr. Erik Sass, MA (Editor at the Economic Standard)
Table 1: Evidence on COVID early treatment therapeutics
| Study # | Author, study title, url link PDF, predominant summary finding on benefit of this drug in the armamentum of early treatment |
| Therapeutic’s name: IVERMECTIN (see Figure 1 and note on ivermectin for inpatient treatment as well as guidance for clinicians, please click here.) | |
| 1) | Espitia-Hernandez G et al. “Effects of Ivermectin-azithromycin-cholecalciferol combined therapy on COVID-19 infected patients: a proof of concept study.” Biomedical Research 2020; 31 (5): 129-133Download PDFSummary: Patients who met inclusion criteria were invited to take Ivermectin (6 mg once daily in day 0,1,7 and 8) plus Azithromycin (500 mg once daily for 4 days) plus Cholecalciferol (4000 UI twice daily for 30 days). Treatment outcome was evaluated on the 10th day onward from the first day of the drug intake. Recovery rate of the 28 patients that received the combination therapy was 100%, the mean symptomatic recovery duration was 3.6 days and negative PCR was confirmed on day 10. |
| 2) | Samaha Ali et al. “Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon.” Viruses 2021 May 26;13(6):989. Doi: 10.3390/v13060989Download PDFSummary: A randomized controlled trial was conducted in 100 asymptomatic Lebanese subjects that tested positive for SARS-CoV2. Fifty patients received standard preventive treatment, mainly supplements, and the experimental group received a single dose according to body weight of ivermectin, in addition to the same supplements the control group received. 72 hours after the regimen started, the increase in Ct-values was dramatically higher in the ivermectin than in the control group. Additionally, more subjects in the control group developed clinical symptoms: three individuals (6%) required hospitalization, compared to 0% for the ivermectin group. |
| 3) | Cadegiani, FA et al. “Early COVID-19 Therapy with Azithromycin Plus Nitazoxanide, Ivermectin or Hydroxychloroquine in Outpatient Settings Significantly Reduced Symptoms Compared to Known Outcomes in Untreated Patients.” New Microbes and New Infections, July 7, 2021. Doi: 10.1016/j.nmni.2021.100915Download PDFSummary: Compared to CG1 and CG2, AG showed a reduction of 31.5 to 36.5% in viral shedding (p < 0.0001), 70 to 85% and 70 to 73% in duration of COVID-19 clinical symptoms… For every 1,000 confirmed cases for COVID-19, a minimum of 140 patients were prevented from hospitalization (p < 0.0001), 50 from mechanical ventilation, and five deaths. |
| 4) | Biber A et al. “Favorable outcome on viral load and culture viability using Ivermectin in early treatment of non-hospitalized patients with mild COVID-19 – A double-blind, randomized placebo-controlled trial.” medRxiv, May 31, 2021. Doi: 10.1101/2021.05.31.21258081Download PDFSummary: The double-blinded trial compared patients receiving ivermectin 0·2 mg/kg for 3 days vs. placebo in non-hospitalized COVID-19 patients… Primary endpoint was reduction of viral-load on the 6th day (third day after termination of treatment) as reflected by Ct level>30 (non-infectious level)… On day 6, 34 out of 47 (72%) patients in the ivermectin arm reached the endpoint, compared to 21/42 (50%) in the placebo arm… Cultures at days 2 to 6 were positive in 3/23 (13.0%) of ivermectin samples vs. 14/29 (48.2%) in the placebo group (p=0.008). |
| 5) | Merino J et al. “Ivermectin and the odds of hospitalization due to COVID-19: evidence from a quasi-experimental analysis based on a public intervention in Mexico City.” SocArXiv, May 3, 2021. Doi: 10.31235/osf.io/r93g4Download PDFSummary: “We estimated logistic-regression models with matched observations adjusting by age, sex, COVID severity, and comorbidities. We found a significant reduction in hospitalizations among patients who received the ivermectin-based medical kit; the range of the effect is 52% – 76% depending on model specification.” |
| 6) | Fonseca SNS et al. “Risk of hospitalization for Covid-19 outpatients treated with various drug regimens in Brazil: Comparative analysis.” Travel Med Infect Dis. 2020 November-December; 38. Doi: 10.1016/j.tmaid.2020.101906Download PDFSummary: “Use of hydroxychloroquine (HCQ), prednisone or both significantly reduced hospitalization risk by 50–60%. Ivermectin, azithromycin and oseltamivir did not substantially reduce risk further.” |
| 7) | Lima-Morales R et al. “Effectiveness of a multidrug therapy consisting of Ivermectin, Azithromycin, Montelukast, and Acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico.” Int J Infect Dis. 2021 Apr; 105: 598-605. Doi: 10.1016/j.ijid.2021.02.014Download PDFSummary: “A comparative effectiveness study was performed among 768 confirmed SARS-CoV-2 cases aged 18-80 years, who received ambulatory care… A total of 481 cases received the TNR4 therapy, while 287 received another treatment (comparison group). Nearly 85% of cases who received the TNR4 recovered within 14 days compared to 59% in the comparison group. The likelihood of recovery within 14 days was 3.4 times greater among the TNR4 group than in the comparison group. Patients treated with TNR4 had a 75% and 81% lower risk of being hospitalized or death, respectively, than the comparison group.” |
| 8) | Loué P et al. “Ivermectin and COVID-19 in Care Home: Case Report.” J Infect Dis Epidemiol. April 17, 2021; 7:4, 202. Doi: 10.23937/2474-3658/1510202Download PDFSummary: “Of the 25 PCR-positive patients, 10 chose to take the IVM treatment (group 1) and 15 chose not to take IVM (group 2). Patients of the group 1 received a single dose of 200 micrograms/kg body weight… Mortality occurred in 1 patient in the group 1 and 5 of the group 2 (p = 0.34).” |
| Therapeutic’s name: DOXYCYCLINE | |
| 1) | Hashim H et al. “Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq.” medRxiv, October 27, 2020. Doi: 10.1101/2020.10.26.20219345Download PDFSummary: Randomized controlled study on 70 COVID-19 patients (48 mild-moderate, 11 severe, and 11 critical patients) treated with 200ug/kg PO of Ivermectin per day for 2-3 days along with 100mg PO doxycycline twice per day for 5-10 days plus standard therapy; the second arm is 70 COVID-19 patients (48 mild-moderate and 22 severe and zero critical patients) on standard therapy… among all patients and among severe patients, 3/70 (4.28%) and 1/11 (9%), respectively progressed to a more advanced stage of the disease in the Ivermectin-Doxycycline group versus 7/70 (10%) and 7/22 (31.81%), respectively in the control group. |
| 2) | Yates P et al. “Doxycycline treatment of high-risk COVID-19-positive patients with comorbid pulmonary disease.” Therapeutic Advances in Respiratory Disease. January 2020. Doi: 10.1177/1753466620951053Download PDFSummary: Case study of four high-risk, symptomatic COVID-19 patients who showed rapid improvement after treatment with doxycycline. |
| 3) | Ahmad I et al. “Doxycycline and Hydroxychloroquine as Treatment for High-Risk COVID-19 Patients: Experience from Case Series of 54 Patients in Long-Term Care Facilities.” medRxiv, May 22, 2020. Doi: 10.1101/2020.05.18.20066902Download PDFSummary: A series of 54 high-risk patients, who developed a sudden onset of fever, cough, and shortness of breath (SOB) and were diagnosed or presumed to have COVID-19, were started with a combination of DOXY-HCQ and 85% (n=46) patients showed clinical recovery defined as: resolution of fever and SOB, or a return to baseline setting if patients are ventilator-dependent. A total of 11% (n=6) patients were transferred to acute care hospitals due to clinical deterioration and 6% (n=3) patients died in the facilities. Naive Indirect Comparison suggests these data were significantly better outcomes than data reported in MMWR for comparable facilities. |
| 4) | Gendrot M et al. “In Vitro Antiviral Activity of Doxycycline against SARS-CoV-2.” Molecules, 2020, 25(21), 5064; Doi: 10.3390/molecules25215064Download PDFSummary: Doxycycline showed in vitro activity on Vero E6 cells infected with a clinically isolated SARS-CoV-2 strain (IHUMI-3) with median effective concentration (EC50) of 4.5 ± 2.9 µM, compatible with oral uptake and intravenous administrations. Doxycycline interacted both on SARS-CoV-2 entry and in replication after virus entry. Besides its in vitro antiviral activity against SARS-CoV-2, doxycycline has anti-inflammatory effects by decreasing the expression of various pro-inflammatory cytokines and could prevent co-infections and superinfections due to broad-spectrum antimicrobial activity. |
| 5) | Meybodi ZA et al. “Effectiveness and Safety Doxycycline in treating COVID-19 Positive Patients: A pilot clinical study.” Pakistan Journal of Medical and Health Sciences, June 2021; 15(1): 610-614. Doi: 10.21203/rs.3.rs-141875/v3Download PDFSummary: Patients who met the inclusion criteria received doxycycline at a dose of 100 mg every 12 hours for seven days and then were evaluated on the baseline day. On days 3, 7, and 14 after admission for cough, shortness of breath, temperature, and oxygen saturation. Finding: Out of 21 patients, 11 patients were male, and ten patients were female. Cough, shortness of breath, temperature, and O2 sat improved in both outpatients and inpatients compared to baseline. |
| Therapeutic’s name: VITAMIN D | |
| 1) | Kaufman H et al. “SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels.” PLOS One, September 17, 2020. Doi: 10.1371/journal.pone.0239252Download PDFSummary: Retrospective, observational analysis to determine if circulating 25-hydroxyvitamin D (25(OH)D) levels are associated with severe acute respiratory disease coronavirus 2 (SARS-CoV-2) positivity rates. A total of 191,779 patients were included, median age 54 years, 68% female. The SARS-CoV-2 positivity rate was higher in the 39,190 patients with “deficient” 25(OH)D values (<20 ng/mL) (12.5%, 95% C.I. 12.2–12.8%) than in the 27,870 patients with “adequate” values (30–34 ng/mL) (8.1%, 95% C.I. 7.8–8.4%) and the 12,321 patients with values ≥55 ng/mL (5.9%, 95% C.I. 5.5–6.4%). |
| 2) | Israel A et al. “The link between vitamin D deficiency and Covid-19 in a large population.” medRxiv, September 7, 2020. Doi: 10.1101/2020.09.04.20188268Download PDFSummary: Population-based study to assess the relationship between prevalence of vitamin D deficiency and COVID-19 incidence. Matched 52,405 infected patients with 524,050 control individuals of the same sex, age, geographical region and used conditional logistic regression to assess the relationship between baseline vitamin D levels, acquisition of vitamin D supplements in the last 4 months, and positive COVID-19. Found highly significant correlation between prevalence of vitamin D deficiency and COVID-19 incidence, and between female-to-male ratio for severe vitamin D deficiency and female-to-male ratio for COVID-19 incidence. In the matched cohort, found significant association between low vitamin D levels and the risk of COVID-19, with the highest risk observed for severe vitamin D deficiency. A significant protective effect was observed for members who acquired liquid vitamin D formulations (drops) in the last 4 months. |
| 3) | Katz J. “Increased risk for COVID-19 in patients with vitamin D deficiency.” Nutrition, 2021 Apr; 84:111106. Doi: 10.1016/j.nut.2020.111106Download PDFSummary: Patients with vitamin D deficiency were 4.6 times more likely to be positive for COVID-19 (indicated by the ICD-10 diagnostic code COVID19) than patients with no deficiency (P < 0.001). In addition, patients with vitamin D deficiency were 5 times more likely to be infected with COVID-19 than patients with no deficiency after adjusting for age groups (OR = 5.155; P < 0.001). |
| 4) | Baktash V et al. “Vitamin D status and outcomes for hospitalised older patients with COVID-19.” Postgrad Med J. 2021 Jul;97(1149):442-447. Doi: 10.1136/postgradmedj-2020-138712Download PDFSummary: Prospective cohort study between 1 March and 30 April 2020 to assess the importance of vitamin D deficiency in older patients with COVID-19. The cohort consisted of patients aged ≥65 years presenting with symptoms consistent with COVID-19 (n=105). COVID-19-positive arm demonstrated lower median serum 25(OH)D level of 27 nmol/L (IQR=20-47 nmol/L) compared with COVID-19-negative arm, with median level of 52 nmol/L (IQR=31.5-71.5 nmol/L) (p value=0.0008). Among patients with vitamin D deficiency, there was higher peak D-dimer level (1914.00 μgFEU/L vs 1268.00 μgFEU/L) (p=0.034) and higher incidence of NIV support and high dependency unit admission (30.77% vs 9.68%) (p=0.042). |
| 5) | Martín Giménez VM et al. “Vitamin D deficiency in African Americans is associated with a high risk of severe disease and mortality by SARS-CoV-2.” Journal of Human Hypertension vol 35, pages 378–380 (2021). Doi: 10.1038/s41371-020-00398-zDownload PDFSummary: Despite the lack of studies to define the adequate level of vitamin D to protect against viral infection, we agree with Grant et al., and estimate that a range between 40 and 60 mg/dL and the recommended dose to achieve this, between 5000 and 10,000 IU/day for several weeks. |
| 6) | Ricci A et al. “Circulating Vitamin D levels status and clinical prognostic indices in COVID-19 patients.” Respiratory Research vol 22, Article number: 76 (2021). Doi: 10.1186/s12931-021-01666-3Download PDFSummary: Vitamin D levels were deficient in (80%) of patients, insufficient in (6.5%) and normal in (13.5%). Patients with very low Vitamin D plasma levels had more elevated D-Dimer values, a more elevated B lymphocyte cell count, a reduction of CD8 + T lymphocytes with a low CD4/CD8 ratio, more compromised clinical findings (measured by LIPI and SOFA scores) and thoracic CT scan involvement. Vitamin D deficiency is associated with compromised inflammatory responses and higher pulmonary involvement in COVID-19 affected patients. |
| 7) | Lakkireddy M et al. “Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID 19 disease.” Scientific Reports vol 11, May 20, 2021. Doi: 10.1038/s41598-021-90189-4Download PDFSummary: Therapeutic improvement in vitamin D to 80–100 ng/ml has significantly reduced the inflammatory markers associated with COVID-19 without any side effects. |
| Therapeutic’s name: ZINC | |
| 1) | Carlucci P et al. “Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients.” Journal of Medical Microbiology, September 15, 2020, v 69 issue 10. Doi: 1099/jmm.0.001250Download PDFSummary: In univariate analyses, zinc sulphate increased the frequency of patients being discharged home, and decreased the need for ventilation, admission to the ICU and mortality or transfer to hospice for patients who were never admitted to the ICU. |
| 2) | Dubourg G et al. “Low blood zinc concentrations in patients with poor clinical outcome during SARS-CoV-2 infection: is there a need to supplement with zinc COVID-19 patients?” Journal of Microbiology, Immunology and Infection, February 13, 2021. 1016/j.jmii.2021.01.012Download PDFSummary:Among 275 patients with COVID-19, we found that median blood zinc level was significantly lower in patients with poor clinical outcome (N=75) as compared to patients with good clinical outcome (N=200) (840 μg/L versus 970 μg/L; p< 0.0001), suggesting that zinc supplementation could be useful for patients with severe COVID-19. |
| 3) | Frontera J et al. “Treatment with Zinc is Associated with Reduced In-Hospital Mortality Among COVID-19 Patients: A Multi-Center Cohort Study.” BMC Infectious Diseases [preprint]. October 26, 2020. Doi: 21203/rs.3.rs-94509/v1Download PDFSummary: Among 3,473 patients (median age 64, 1947 [56%] male, 522 [15%] ventilated, 545[16%] died), 1,006 (29%) received Zn+ionophore. Zn+ionophore was associated with a 24% reduced risk of in-hospital mortality (12% of those who received Zn+ionophore died versus 17% who did not). |
| 4) | Heller RA et al. “Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker.” Redox Biology, January 2021, v 38. Doi: 1016/j.redox.2020.101764Download PDFSummary: Our data indicate a profound and acute zinc deficiency in the majority of patients with COVID-19 when admitted to the hospital. … We conclude that Zn and SELENOP status within the reference ranges indicate high survival odds in COVID-19, and assume that correcting a diagnostically proven deficit in Se and/or Zn by a personalised supplementation may support convalescence. |
| 5) | Vogel-González M et al. “Low zinc levels at clinical admission associates with poor outcomes in COVID-19.” medRxiv, October 11, 2020. Doi: 1101/2020.10.07.20208645Download PDFSummary: Individuals with SZC at admission <50 µg/dl had a mortality of 21% that was significantly higher compared with 5% mortality in individuals with zinc at admission ≥50 µg/dl; p<0·001. Our study demonstrates a correlation between serum zinc levels and COVID-19 outcome. Serum zinc levels lower than 50 mcgg/dl at admission correlated with worse clinical presentation, longer time to reach stability and higher mortality. |
| 6) | Jothimani D et al. “COVID-19: Poor outcomes in patients with zinc deficiency.” International Journal of Infection Diseases, November 2020, v 100: 343-349. Doi: 1016/j.ijid.2020.09.014Download PDFSummary: More patients in the Zinc deficient group … required ICU care (7 vs 2, P=0.266) and recorded deaths (5 vs 0) in comparison to patients with normal Zinc levels. |
| 7) | Yasui Y et al. “Analysis of the predictive factors for a critical illness of COVID-19 during treatment - relationship between serum zinc level and critical illness of COVID-19.” International Journal of Infectious Diseases, November 2020, v 100: 230-236. Doi: 1016/j.ijid.2020.09.008Download PDFSummary:Based on the measurement results of serum zinc levels in patients with COVID-19 in our hospital, almost all severe cases showed subclinical or clinical zinc deficiency. Prolonged hypozincemia was found to be a risk factor for a severe case of COVID-19. In evaluating the relationship between the serum zinc level and severity of patients with COVID-19 by multivariate logistic regression analysis, critical illness can be predicted through the sensitivity and false specificity of an ROC curve with an error rate of 10.3% and AUC of 94.2% by only two factors: serum zinc value (P = 0.020) and LDH value (P = 0.026). |
| 8) | Derwand R et al. “COVID-19 outpatients: early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: a retrospective case series study.” International Journal of Antimicrobial Agents, December 2020, v 56:6. Doi: 1016/j.ijantimicag.2020.106214Download PDFSummary: After 4 days (median, IQR 3-6, available for N=66/141) of onset of symptoms, 141 patients (median age 58 years, IQR 40-67; 73% male) received a prescription for the triple therapy for 5 days. Independent public reference data from 377 confirmed COVID-19 patients of the same community were used as untreated control. 4 of 141 treated patients (2.8%) were hospitalized, which was significantly less (p<0.001) compared with 58 of 377 untreated patients (15.4%) (odds ratio 0.16, 95% CI 0.06-0.5). One patient (0.7%) died in the treatment group versus 13 patients (3.5%) in the untreated group (odds ratio 0.2, 95% CI 0.03-1.5; p=0.12). |
| Therapeutic’s name: COLCHICINE | |
| 1) | Tardif JC et al. “Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial.” Lancet Respir Med. 2021 May 27; Doi: 10.1016/S2213-2600(21)00222-8Download PDFSummary: 2,235 patients were randomly assigned to colchicine and 2,253 to placebo. Among patients with PCR-confirmed COVID-19, colchicine led to a lower rate of the composite of death or hospital admission than placebo. |
| 2) | Scarsi M et al. “Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome.” Ann Rheum Dis. 2020 Oct; 79(10): 1286–1289. Doi: 10.1136/annrheumdis-2020-217712Download PDFSummary: 140 consecutive inpatients were treated with standard of care (hydroxychloroquine and/or intravenous dexamethasone; and/or lopinavir/ritonavir). They were compared with 122 consecutive inpatients treated with colchicine and standard of care (antiviral drugs were stopped before colchicine, due to potential interaction). Patients treated with colchicine had a better survival rate as compared with SoC at 21 days of follow-up (84.2% vs 63.6%). |
| Therapeutic’s name: BROMHEXINE | |
| 1) | Ansarin et al. “Effect of bromhexine on clinical outcomes and mortality in COVID-19 patients: A randomized clinical trial.” BioImpacts, 2020, 10(4), 209-215. Doi: 10.34172/bi.2021.30Download PDFSummary: A total of 78 patients with similar demographic and disease characteristics were enrolled. There was a significant reduction in ICU admissions (2 out of 39 vs. 11 out of 39, P=0.006), intubation (1 out of 39 vs. 9 out of 39, P=0.007) and death (0 vs. 5, P=0.027) in the bromhexine treated group compared to the standard group. No patients were withdrawn from the study because of adverse effects. |
| 2) | Li et al. “Bromhexine Hydrochloride Tablets for the Treatment of Moderate COVID-19: An Open-Label Randomized Controlled Pilot Study.” Clin. Transl. Sci (2020) 13, 1096–1102. Doi: 10.1111/cts.12881Download PDFSummary: A total of 18 patients with moderate COVID-19 were randomized into the BRH group (n = 12) or the control group (n = 6). There were suggestions of BRH advantage over placebo in improved chest computed tomography, need for oxygen therapy, and discharge rate within 20 days. |
| 3) | Maggio et al. “Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS-CoV-2 infection.” Pharmacological Research 157 (July 2020) 104837 Doi: 10.1016/j.phrs.2020.104837Summary: Pharmacokinetic data support the testing of bromhexine use for this indication since, in pulmonary and bronchial epithelial cells, it may reach concentrations 4 to 6-fold higher than those found in the plasma, high enough in principle to inhibit TMPRSS2. |
| 4) | Mareev, et al. “Results of an open, prospective, controlled, comparative study for the treatment of novel coronavirus infection (COVID-19): Bromhexine And Spironolactone for the treatment of Corona Viral Infection Requiring Hospitalization (BISQUIT).” Kardiologiia, 2020;60(11). DOI: 10.18087/cardio.2020.11.n1440English translation: https://pubmed.ncbi.nlm.nih.gov/33487145/Download PDFSummary: 103 patients were included (33 in the bromhexine and spironolactone group and 70 in the control group). Analysis for the group as a whole revealed a statistically significant reduction in hospitalization time from 10.4 to 9.0 days and fever time from 6.5 to 3.9 days. |
| 5) | Mikhaylov, et al. “Bromhexine Hydrochloride Prophylaxis of COVID-19 for Medical Personnel: A Randomized Open-Label Study.” medRxiv preprint, May 29, 2021. Doi: 10.1101/2021.03.03.21252855Download PDFSummary: 25 healthcare workers were assigned to bromhexine hydrochloride treatment (8 mg 3 times per day), and 25 were controls. Fewer participants developed symptomatic COVID-19 in the treatment group compared to controls (0/25 vs 5/25). |
| 6) | Ou, et al. “Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2.” PLOS Pathogens, January 19, 2021. Doi: 10.1371/journal.ppat.1009212Download PDF (from PLOS website)Summary: We show that combinations of hydroxychloroquine and a clinically tested TMPRSS2 inhibitor work together to effectively inhibit SARS-CoV-2 entry. |
| Therapeutic’s name: BUDESONIDE | |
| 1) | Ramakrishnan S et al. “Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial.” Lancet Respir Med, April 9, 2021. Doi: 10.1016/ S2213-2600(21)00171-5Download PDFSummary: 146 participants were randomly assigned, 73 to usual care and 73 to budesonide. For the per-protocol population (n=139), the primary outcome occurred in ten (14%) of 70 participants in the usual care group and one (1%) of 69 participants in the budesonide group. For the ITT population, the primary outcome occurred in 11 (15%) participants in the usual care group and two (3%) participants in the budesonide group. Clinical recovery was 1 day shorter in the budesonide group compared with the usual care group (median 7 days versus 8). The mean proportion of days with a fever in the first 14 days was lower in the budesonide group than the usual care group (2% versus 8%) and the proportion of participants with at least 1 day of fever was lower in the budesonide group when compared with the usual care group. Fewer participants randomly assigned to budesonide had persistent symptoms at days 14 and 28. |
| Therapeutic’s name: DEXAMETHASONE | |
| 1) | Tomazini BM et al. “Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19The CoDEX Randomized Clinical Trial.” JAMA, September 2, 2020. Doi: 10.1001/jama.2020.17021Download PDFSummary: In this randomized clinical trial that included 299 patients, the number of days alive and free from mechanical ventilation during the first 28 days was significantly higher among patients treated with dexamethasone plus standard care when compared with standard care alone (6.6 days vs 4.0 days). |
| 2) | Horby P et al. (RECOVERY Collaborative). “Dexamethasone in Hospitalized Patients with COVID-19.” NEJM, February 25, 2021. Doi: 10.1056/NEJMoa2021436Download PDFSummary: In patients hospitalized with Covid-19, the use of dexamethasone resulted in lower 28-day mortality among those who were receiving either invasive mechanical ventilation or oxygen alone at randomization but not among those receiving no respiratory support. |
| Therapeutic’s name: MONOCLONAL ANTIBODIES | |
| 1) | Verderese JP et al. “Neutralizing Monoclonal Antibody Treatment Reduces Hospitalization for Mild and Moderate Coronavirus Disease 2019 (COVID-19): A Real-World Experience.” Clinical Infectious Diseases, June 24, 2021. Doi: 10.1093/cid/ciab579Download PDFSummary: 707 confirmed COVID-19 patients received NmAbs and 1709 historic COVID-19 controls were included; 553 (78%) received BAM, 154 (22%) received REGN-COV2. Patients receiving NmAb infusion had significantly lower hospitalization rates (5.8% vs 11.4%, P < .0001), shorter length of stay if hospitalized (mean, 5.2 vs 7.4 days; P = .02), and fewer ED visits within 30 days post-index (8.1% vs 12.3%, P = .003) than controls. |
| 2) | O’Brien MP et al. “Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19.” NEJM, August 4, 2021. Doi: 10.1056/NEJMoa2109682Download PDFSummary: Symptomatic SARS-CoV-2 infection developed in 11 of 753 participants in the REGEN-COV group (1.5%) and in 59 of 752 participants in the placebo group (7.8%) (relative risk reduction [1 minus the relative risk], 81.4%; P<0.001). In weeks 2 to 4, a total of 2 of 753 participants in the REGEN-COV group (0.3%) and 27 of 752 par- ticipants in the placebo group (3.6%) had symptomatic SARS-CoV-2 infection (relative risk reduction, 92.6%). REGEN-COV also prevented symptomatic and asymptomatic infections overall (relative risk reduction, 66.4%). Among symptomatic infected par- ticipants, the median time to resolution of symptoms was 2 weeks shorter with REGEN-COV than with placebo (1.2 weeks and 3.2 weeks, respectively), and the duration of a high viral load (>104 copies per milliliter) was shorter (0.4 weeks and 1.3 weeks, respectively). No dose-limiting toxic effects of REGEN-COV were noted. |
| Therapeutic’s name: QUERCETIN | |
| 1) | Di Pierro F et al. “Possible Therapeutic Effects of Adjuvant Quercetin Supplementation Against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study.” Int J General Med, June 8, 2021. Doi: 10.2147/IJGM.S318720Download PDFSummary: Prospective, randomized, controlled, and open-label study. Daily dose of 1000 mg of QP was investigated for 30 days in 152 COVID-19 outpatients to disclose its adjuvant effect in treating the early symptoms and in preventing the severe outcomes of the disease. The results revealed a reduction in frequency and length of hospitalization, in need of non-invasive oxygen therapy, in progression to intensive care units and in number of deaths. The results also confirmed the very high safety profile of quercetin. |
| Therapeutic’s name: FLUVOXAMINE | |
| 1) | Lenze E et al. “Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19. A Randomized Clinical Trial.” JAMA. 2020; 324(22): 2292-2300. Doi: 10.1001/jama.2020.22760Summary: In this randomized trial that included 152 adult outpatients with confirmed COVID-19 and symptom onset within 7 days, clinical deterioration occurred in 0 patients treated with fluvoxamine vs 6 (8.3%) patients treated with placebo over 15 days, a difference that was statistically significant. |
| 2) | Reis G et al. “Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial.” Lancet Global Health. October 27, 2021; 10(1): E42-E51. Doi: 10.1016/S2214-109X(21)00448-4Summary: The proportion of patients observed in a COVID-19 emergency setting for more than 6 h or transferred to a teritary hospital due to COVID-19 was lower for the fluvoxamine group compared with placebo (79 [11%] of 741 vs 119 [16%] of 756) [. . .] There were 17 deaths in the fluvoxamine group and 25 deaths in the placebo group in the primary intention-to-treat analysis (odds ratio [OR] 0·68, 95% CI: 0·36–1·27). There was one death in the fluvoxamine group and 12 in the placebo group for the per-protocol population (OR 0·09; 95% CI 0·01–0·47). |
| 3) | Seftel D et al. “Prospective Cohort of Fluvoxamine for Early Treatment of Coronavirus Disease 19.” Open Forum Infectious Diseases, Volume 8, Issue 2, February 2021. Doi: 10.1093/ofid/ofab050Download PDFSummary: Incidence of hospitalization was 0% (0 of 65) with fluvoxamine and 12.5% (6 of 48) with observation alone. At 14 days, residual symptoms persisted in 0% (0 of 65) with fluvoxamine and 60% (29 of 48) with observation. |
| Therapeutic’s name: PREDNISONE | |
| 1) | Ooi ST et al. “Antivirals With Adjunctive Corticosteroids Prevent Clinical Progression of Early Coronavirus 2019 Pneumonia: A Retrospective Cohort Study.” Travel Open Forum Infectious Diseases, Volume 7, Issue 11, November 2020, ofaa486. Doi: 10.1093/ofid/ofaa486Download PDFSummary: “A combination of corticosteroids and antivirals was associated with lower risk of clinical progression and invasive mechanical ventilation or death in early COVID-19 pneumonia.” |
| 2) | Fonseca SNS et al. “Risk of hospitalization for Covid-19 outpatients treated with various drug regimens in Brazil: Comparative analysis.” Travel Med Infect Dis. 2020 November-December; 38. Doi: 10.1016/j.tmaid.2020.101906Download PDFSummary: “Use of hydroxychloroquine (HCQ), prednisone or both significantly reduced hospitalization risk by 50–60%.” |
| Therapeutic’s name: AZITHROMYCIN | |
| 1) | Taieb F et al. “Hydroxychloroquine and Azithromycin Treatment of Hospitalized Patients Infected with SARS-CoV-2 in Senegal from March to October 2020.” J Clin Med, 2021 Jun 30;10(13):2954. Doi: 3390/jcm10132954.Download PDFSummary: A total of 926 patients were included in this analysis. Six hundred seventy-four (674) (72.8%) patients received a combination of HCQ and AZM. Results showed that the proportion of patient discharge at D15 was significantly higher for patients receiving HCQ plus AZM (OR: 1.63, IC 95% (1.09-2.43). |
| 2) | Lagier JC et al. “Outcomes of 2,111 COVID-19 hospitalised patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a monocentric retrospective analysis.” IHU-Méditerranée Infection [preprint], June 4, 2021.Download PDFSummary: Treatment with HCQ-AZ was an independent protective factor against death – Zinc was independently protective against death in patients treated with HCQ-AZ. |
| 3) | Heras E et al. “COVID-19 mortality risk factors in older people in a long-term care center.” European Geriatric Medicine, November 27, 2020, v 12, p 601–607. Doi: 1007/s41999-020-00432-wDownload PDFSummary: Among 100 COVID-19+ nursing home patients in Andorra, multivariate logistic regression analysis identified hydroxychloroquine plus azithromycin treatment as an independent factor favoring survival compared with no treatment or other treatments. |
| 4) | Ly TDA et al. “Pattern of SARS-CoV-2 infection among dependent elderly residents living in long-term care facilities in Marseille, France, March-June 2020.” Int J Antimicrob Agents, 2020 Dec;56(6):106219. Doi: 1016/j.ijantimicag.2020.106219Summary: Data from 1,691 elderly residents and 1,000 members of staff were retrospectively collected through interviewing the medical teams in 24 LTCFs and using the hospitals’ electronic health recording systems. 116 (51.4%) patients received a course of oral hydroxychloroquine and azithromycin (HCQAZM) for ≥3 days, and 47 (20.8%) died. Through multivariate analysis, the death rate was positively associated with being male (30.7%, vs. 14.0%, OR=3.95, p=0.002), being older than 85 years (26.1%, vs. 15.6%, OR=2.43, p=0.041), and receiving oxygen therapy (39.0%, vs. 12.9%, OR=5.16, p<0.001) and negatively associated with being diagnosed through mass screening (16.9%, vs. 40.5%, OR=0.20, p=0.001) and receiving HCQ-AZM treatment ≥3 days (15.5%, vs. 26.4%, OR=0.37, p=0.02). |
| 5) | Lauriola M et al. “Effect of combination therapy of hydroxychloroquine and azithromycin on mortality in COVID‐19 patients.” Clinical and Translational Science, September 14, 2020. Doi: 1111/cts.12860Download PDFSummary: In this study, we found a reduced in‐hospital mortality in patients treated with a combination of hydroxychloroquine and azithromycin after adjustment for comorbidities. … At multivariable Cox proportional hazard regression analysis, … use of hydroxychloroquine + azithromycin (vs. no treatment) (HR 0.265, 95%CI 0.171‐0.412, p<0.001) was inversely associated [with death]. |
| 6) | Arshad S et al. “Treatment with Hydroxychloroquine, Azithromycin, and Combination in Patients Hospitalized with COVID-19.” Int Jour Inf Dis, July 1, 2020, 97: 396-403. Doi: 10.1016/j.ijid.2020.06.099Download PDFSummary: In this multi-hospital assessment, when controlling for COVID-19 risk factors, treatment with hydroxychloroquine alone and in combination with azithromycin was associated with reduction in COVID-19 associated mortality. |
| Therapeutic’s name: HYDROXYCHLOROQUINE (Figure 2) | |
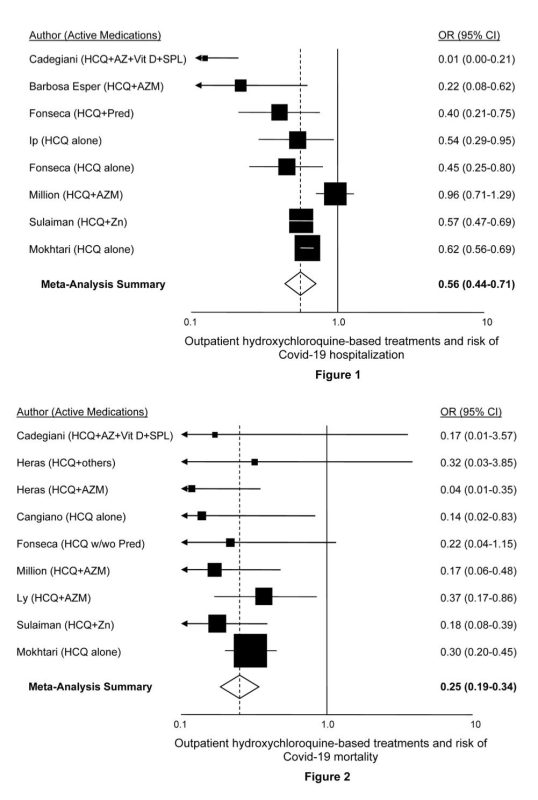
| 1) | Risch, Harvey. “Hydroxychloroquine in Early Treatment of High-Risk COVID-19 Outpatients: Efficacy and Safety Evidence.” Sixth version, updated June 17, 2021.Download PDFSummary: Every study of high-risk outpatient hydroxychloroquine (HCQ) use has shown risk reduction for hospitalization or mortality. Meta-analysis demonstrates 40% reduction in hospitalization and 75% reduction in mortality. A large database study of more than 900,000 older patients taking hydroxychloroquine shows no excess all-cause mortality and no excess occurrence of fatal cardiac arrhythmia. |
| 2) | Million M et al. “Early treatment with hydroxychloroquine and azithromycin in 10,429 COVID-19 outpatients: A monocentric retrospective cohort study.” Accepted for publication, Int J Infect Dis.Download PDFSummary: Cohort of 10,429 COVID-19 patients treated with HCQ, azithromycin and other medications. Among patients age 60 and older, 1,495 patients treated with HCQ+azithromycin for 3+ days were compared to 520 patients given the medications for less than 3 days, or given only the individual medications, or not given either one. The age, sex and time-period adjusted-regression analysis showed a mortality odds ratio of 0.17. |
| 3) | Mokhtari M et al. “Clinical outcomes of patients with mild COVID-19 following treatment with hydroxychloroquine in an outpatient setting. Int Immunopharmacol Vol 96, July 2021. Doi: 10.1016/j.intimp.2021.107636Download PDFSummary: Multicenter, population-based national retrospective-cohort investigation of 28,759 adults with mild COVID-19 seen within 7 days of symptom onset between March and September 2020 in Iran. Treatment with HCQ was associated with a 38% reduction in risk of hospitalization and a 70% reduction in mortality risk, both highly statistically significant. |
| 4) | Barbosa Esper, et al. “Empirical treatment with hydroxychloroquine and azithromycin for suspected cases of COVID-19 followed-up by telemedicine.” April 15, 2020. Accessed April 30, 2020.Download PDFSummary: Even though the severities of symptoms and comorbidities were substantially greater in the treated patients than the controls, the need for hospitalization was significantly lower among those receiving hydroxychloroquine: 1.2% in patients starting treatment before day 7 of symptoms and 3.2% for patients starting treatment after day 7, compared to 5.4% for controls. No cardiac arrhythmias were reported in the 412 treated patients. |
| 5) | Szente Fonseca SN et al. “Risk of hospitalization for Covid-19 outpatients treated with various drug regimens in Brazil: Comparative analysis.” Travel Med Infect Dis 2020;38:101906. Doi: 10.1016/j.tmaid.2020.101906Download PDFSummary: Study of 717 tested-positive symptomatic patients over age 40, mean age 51, presenting between May 11 and June 3, 2020 in Brazil. Adjusted for age, gender, dyspnea at presentation, obesity, diabetes, and heart disease, use of both HCQ and prednisone together was associated with an odds ratio for hospitalization of 0.40; use of HCQ only, odds ratio=0.45; and use of prednisone only, odds ratio=0.51. |
| 6) | Ip A et al. “Hydroxychloroquine in the treatment of outpatients with mildly symptomatic COVID-19: A multi-center observational study. BMC Infect Dis 2021;21:72. Doi: 10.1186/s12879-021-05773-wDownload PDFSummary: Between March 1 and April 22, 2020, 1,274 patients with non-admission ER visits were identified and confirmed infected with SARS-CoV-2 by PCR testing. 97 received prescriptions for or had started taking HCQ, and from the remaining 1,177, 970 were propensity-score matched by age, demographic variables and a host of comorbidity factors, presenting symptoms, indicators of disease severity, baseline laboratory tests, and ER-visit and follow-up times. More than three-quarters of the subjects had comorbidities or were over age 60, making them high-risk. In the matched multivariate analysis, treatment with HCQ significantly cut the risk of hospitalization by 47%. |
| 7) | Ly TDA et al. “Pattern of SARS-CoV-2 infection among dependant elderly residents living in long-term care facilities in Marseille, France, March-June 2020.” Int J Antimicrob Agents 2020;56(6):106219. Doi: 10.1016/j.ijantimicag.2020.106219Download PDFSummary: Study of 23 nursing homes in Marseille, France in which of 226 infected residents, 37 were detected because of COVID-19 symptoms and 189 through mass screening. In multivariate analysis adjusted for sex, age, use of oxygen therapy and detection modality (symptoms vs screening), receipt of HCQ+azithromycin for at least three days was associated with 63% reduced mortality risk. |
| 8) | Heras E et al. “COVID-19 mortality risk factors in older people in a long-term care center.” Eur Geriatr Med 2021;12(3):601-607. Doi: 10.1007/s41999-020-00432-wDownload PDFSummary: Study identified 100 PCR-confirmed COVID-19 patients, median age 85, who received HCQ+azithromycin, HCQ with other antibiotics such as beta-lactam or quinolone types, or other antibiotics alone. In multivariate analysis of risk-adjusted mortality, treatment with HCQ+azithromycin vs. only other antibiotics had OR=0.044; treatment with HCQ+other antibiotics vs. other antibiotics alone had OR=0.32. |
| 9) | Cangiano B et al. “Mortality in an Italian nursing home during COVID-19 pandemic: correlation with gender, age, ADL, vitamin D supplementation, and limitations of the diagnostic tests.” Aging 2020;12. Doi: 10.18632/aging.202307Download PDFSummary: Ninety-eight of 157 residents in a nursing home in Milan, Italy, average age 90, tested positive for SARS-CoV-2. In logistic regression models adjusted for age, sex, Barthel’s index and BMI, receipt of HCQ was associated with 7-fold reduced mortality. |
| 10) | Sulaiman T et al. “The effect of early hydroxychloroquine-based therapy in COVID-19 patients in ambulatory care settings: A nationwide prospective cohort study.” Preprints 2020. Doi: 10.1101/2020.09.09.20184143Download PDFSummary: Roughly 8,000 mild-moderate cases of PCR-positive COVID-19 presenting at national outpatient treatment clinics in Saudi Arabia between 5-26 June, 2020 were recruited for enrollment. Treated and control patients were comparable in distributions of age, sex and nine comorbidities reported. In multivariate modeling adjusted for age, gender and comorbidities, HCQ receipt cut mortality 3-fold, while there was a 5-fold reduction in mortality with HCQ+zinc treatment vs zinc only. |
| 11) | Cadegiani, FA et al. “Early COVID-19 Therapy with Azithromycin Plus Nitazoxanide, Ivermectin or Hydroxychloroquine in Outpatient Settings Significantly Reduced Symptoms Compared to Known Outcomes in Untreated Patients.” New Microbes and New Infections, July 7, 2021. Doi: 1016/j.nmni.2021.100915Download PDFSummary: In total, 159 patients were treated with HCQ and 137 controls participated. There were no hospitalizations or deaths among the HCQ patients, whereas 27 control patients were hospitalized and 2 died. |
Figure 1: Studies of ivermectin as an outpatient treatment
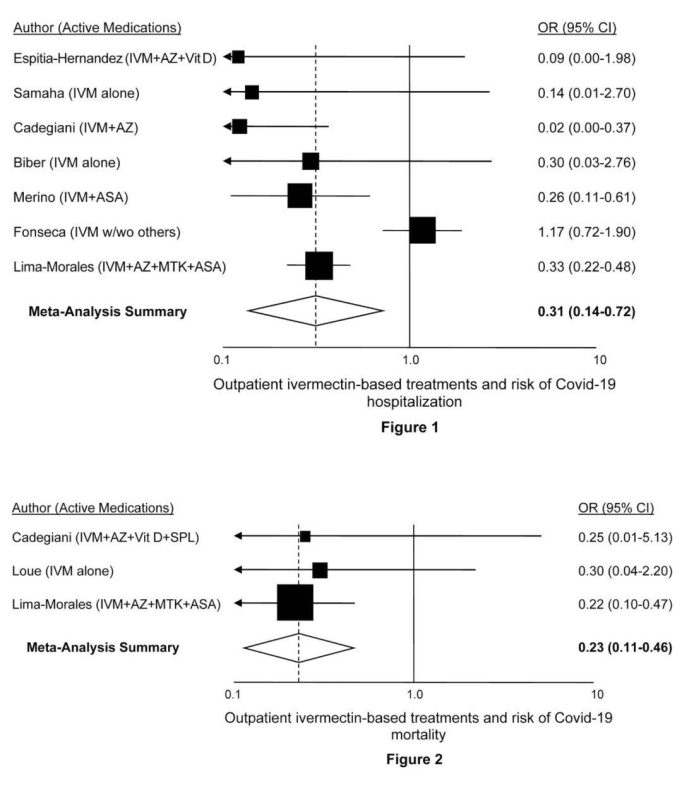
Figure 2: Studies of hydroxychloroquine as an outpatient treatment
Published under a Creative Commons Attribution 4.0 International License
For reprints, please set the canonical link back to the original Brownstone Institute Article and Author.









