Already missing the Covid-19 pandemic? Well, fear not. The German-EU “VACCELERATE” programme is already on the lookout for the next “pathogen with pandemic potential,” as authors affiliated with the programme put it in a new paper titled “Predicting the next pandemic.”
The paper is devoted to a survey of the VACCELERATE consortium’s own members on the pathogen most likely to produce the “next pandemic” – or the next “potential pandemic generator,” as the authors also put it. The responses involved a ranking of the various candidates and were tallied using a points system, much like the Eurovision song contest.
And the winner is…
Influenza! The flu was named by nearly 80% of respondents and garnered nearly one-third of the points. A hitherto unknown “Disease X” finished second, and the Coronaviruses, SARS-CoV-2 and SAR-Cov-1, finished third and fourth respectively. The full results are reproduced below.
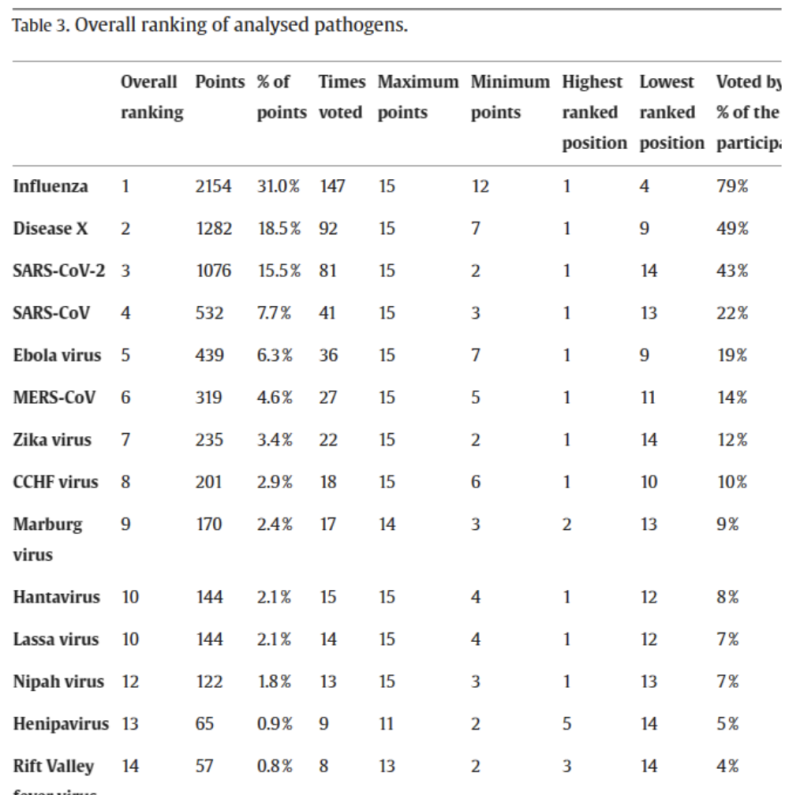

According to the project description on the funding page, the programme’s original purpose, in keeping with the objective of EUVAP, was to create an EU-wide network of clinical trial sites and a registry of willing trial participants, in order to fast-track specifically Covid-19 vaccine candidates. “The ongoing COVID-19 pandemic creates an unprecedented burden worldwide” the description reads,
Vaccine-induced immunity is the only promising solution. There is continued need for phase 2 & 3 vaccine trials to reach long-term, large-scale immunity of the entire European population. VACCELERATE will be the pan-European backbone accelerating phase 2 & 3 COVID-19 vaccine trials.
With COVID-19 vaccines having already been rolled out in record time shortly before its launch, by the time EUVAP had morphed into VACCELERATE in 2021, this original purpose was, of course, largely obsolete. Hence, the programme’s pivot toward the next “pathogen with pandemic potential” is hardly surprising.
Indeed, the original project description already notes that “Beyond the COVID-19 pandemic, [VACCELERATE] will be an established pandemic preparedness network, ready to face emerging future pandemics” and “enhance vaccine development capacity in Europe.”
The VACCELERATE website notes, moreover, that the programme “is funded by the European Commission’s activities for future pandemic preparedness, the HERA Incubator.” HERA is the EU’s Health Emergency preparedness and Response Authority, which was likewise created in 2021.
The VACCELERATE consortium is led by the Clinical Trial Unit of the German Center for Infection Research (DZIF). The Clinical Trial Unit is based at the University of Cologne.
The DZIF is a German public agency which partners with pharmaceutical companies in developing vaccines. One of the DZIF’s partners is none other than BioNTech. See the screenshot from the DZIF website below. BioNTech is the German developer and in fact legal manufacturer of what is more commonly known as the “Pfizer” vaccine. Pfizer performs (some) manufacturing activities as a contract manufacturer on BioNTech’s behalf (see here). BioNTech has also earned far more than Pfizer on sales of the drug.

The head of the DZIF’s Product Development Unit is none other than Klaus Cichutek, who is at the same time the president of the German vaccine regulator, the PEI or Paul Ehrlich Institute (so-named for the German immunologist, not the American population control theorist).
It is this dual role of enabler and regulator which raises obvious questions about the impartiality of the PEI’s oversight of the BioNTech vaccine, and these questions are all the thornier given the leading role which, by Cichutek’s own admission, the PEI plays at the European Medicines Agency (EMA).
Oliver Cornely of the University of Cologne is both the VACCELERATE project leader and the coordinator of the DZIF’s Clinical Trial Unit. Cornely and Cichutek are pictured below in the DZIF’s 2020 annual report.


Republished from The Daily Sceptic
Join the conversation:


Published under a Creative Commons Attribution 4.0 International License
For reprints, please set the canonical link back to the original Brownstone Institute Article and Author.









