“Toxic and Effective” Does Not Make for a Snappy Marketing Slogan
Over the last 4+ years, we have heard the slogan “safe and effective” ad nauseum in relation to vaccines — repeated by health agencies, media, and pharmaceutical companies. But what does “safe” really mean?
This post isn’t about fear. It’s about precision.
The word “safe” is not scientific, especially when evaluating the effects of substances, such as vaccines, on living organisms. Every substance that is taken into the body, either by ingesting or injection, can be toxic, even water and oxygen.
Toxicity is not binary.
Substances aren’t simply “toxic” or “non-toxic” — toxicity is measured on a spectrum.
The same logic applies to vaccines: they contain active and inactive components that should be assessed across a spectrum of toxicity.
“What is it that is not poison? All things are poison and nothing is without poison. It is the dose only that makes a thing not a poison.”
–Paracelsus (1493-1541)
Toxicity: An Overview
Toxicity refers to the inherent capacity of a substance to cause harmful effects on living organisms and the degree to which a substance can harm living organisms. It can affect whole organisms, like humans, animals, plants, and microorganisms, or specific parts of them, such as cells or organs. For example, neurotoxicity is damage to the nervous system from exposure to a substance, or reproductive toxicity, the adverse effects on sexual function, fertility, or offspring.
The toxicity of a chemical is referred to numerically using the term Lethal Dose 50 (LD50). The LD50 describes the amount of chemical ingested or absorbed by the skin in test animals that causes death in 50% of the test animals used during a toxicity test study.
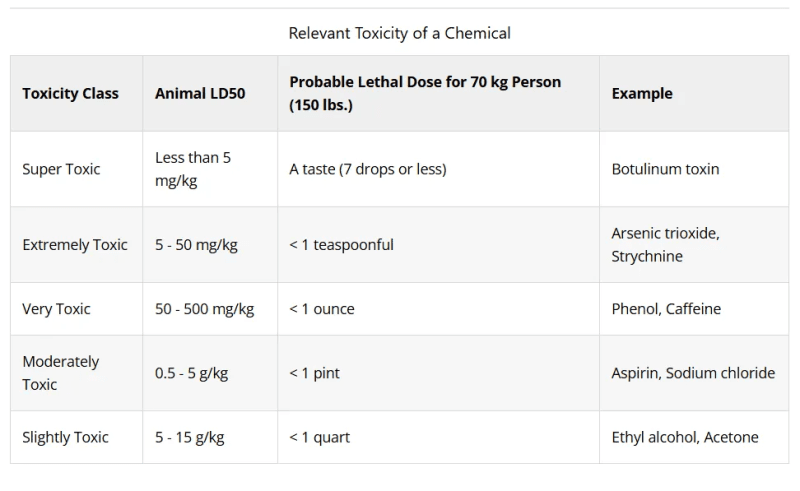

Another common term is Lethal Concentration 50 (LC50), which describes the amount of chemical inhaled by test animals that causes death in 50% of test animals used during a toxicity test study. The lower the LD50 or LC50 number, the more toxic the chemical.
There are a number of factors that influence the toxic effects of chemicals on the body. These include, but are not limited to:
- The quantity and concentration of the chemical. Even typically harmless substances can be toxic at high doses (e.g., water intoxication), while highly toxic substances may have no detectable effects at low doses (e.g., snake venom).
- The length of time and the frequency of the exposure.
- The route of the exposure. Substances can enter the body through inhalation (breathing), ingestion (swallowing), or direct contact with the skin or eyes.
- If mixtures of chemicals are involved.
- Genetics, age, health status of the individual, and environment can affect how someone responds to any medical product — including vaccines.
Toxic effects are generally classified as acute toxicity or chronic toxicity.
- Acute toxicity is generally thought of as a single, short-term exposure where effects appear immediately and are often reversible. An example of acute toxicity relates to the overconsumption of alcohol and “hangovers.”
- Chronic toxicity is generally thought of as frequent exposures where effects may be delayed (even for years) and are generally irreversible. Chronic toxicity can also result in acute exposures, with long-term chronic effects. An example of chronic toxicity relates to cigarette smoking and lung cancer.
Toxicology studies aim to define the limits of toxicity, determining how much exposure can be tolerated before adverse effects appear. This often involves identifying target organs, evaluating dose-response relationships, and assessing the potential for recovery.
Vaccines and Toxicity: A Measurable Spectrum
A Proposed Framework: Rating Vaccines on a Spectrum of Toxicity
Current vaccine safety evaluations focus on overall adverse events and statistical safety profiles. But what if we had a transparent, ingredient-level, biology-based toxicity index — a way to give each vaccine a score based on measurable toxicological data?
Instead of saying vaccines are “safe and effective” we could rate vaccines with a score for toxicity based on its toxicological profile.
To rate vaccines for toxicity, we need a multifactorial scoring system that considers both chemical and biological toxicity potential — tailored to both the general population and sensitive subgroups.
Not to undermine vaccines, but to make safety science more rigorous, more transparent, and more personalized.
1. Ingredient-Based Toxicity Scoring
Each component of a vaccine — adjuvants, preservatives, stabilizers, and residuals — has a known toxicological profile.
Score based on the known toxicological profiles of each component (per dose):
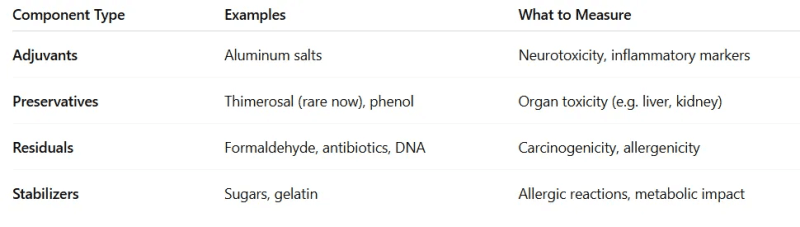

Each compound could receive a score based on:
- Known LD50 (lethal dose in animals)
- Human safety thresholds (e.g., EPA, FDA)
- Accumulation potential
- Dose per vaccine
Example: 0–5 score per compound, where 0 = biologically inert, 5 = known to have toxic effects at low doses
2. Biological Response Profile
This score reflects how the body reacts biologically to the vaccine. Measure and score biological effects, post-vaccination, in clinical or experimental settings:
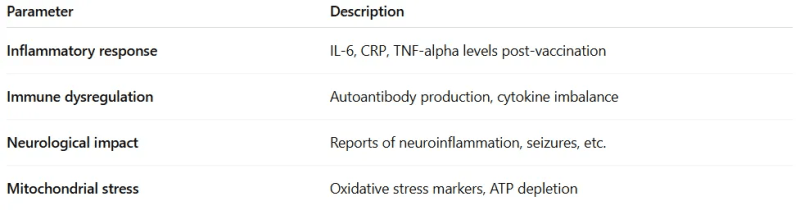

Studies could be conducted using lab biomarkers or long-term health monitoring. These are measurable in lab settings or clinical trials and can be used to detect subclinical toxicity.
3. Population-Level Adverse Event Rate
Real-world safety matters!
This category scores the rate and severity of reported adverse events using real-world data from pharmacovigilance systems (VAERS, EudraVigilance, etc.):
- Rate of serious adverse events per million doses
- Type of events (neurological, autoimmune, allergic)
- Stratified by age, sex, and comorbidities
We would need to adjust for underreporting and confounding, but trends can be identified.
Example metric: Rate of SAE (serious adverse events) per million doses, weighted by severity (mild, serious, fatal).
4. Cumulative Toxic Load
Some vaccines are given in series (e.g., infant schedule). Score cumulative exposure across time.
- Total aluminum exposure from series (µg) across a schedule
- Number of antigens or excipients over schedule
- Adjuvant or preservative stacking from combination vaccines
- Time between doses (e.g., immune system stress from back-to-back shots)
5. Sensitive Population Consideration
Some groups are more vulnerable to certain toxic effects:
- Infants and toddlers (developing immune and neurological systems)
- Pregnant women
- People with autoimmune or neurological conditions
- Individuals with impaired detoxification pathways (e.g., MTHFR variants)
Vaccines should be scored for risk level in these groups and adjusted accordingly
A Hypothetical “Toxicity Index”
Each vaccine could be scored on a 0–100 scale, made from weighted categories:
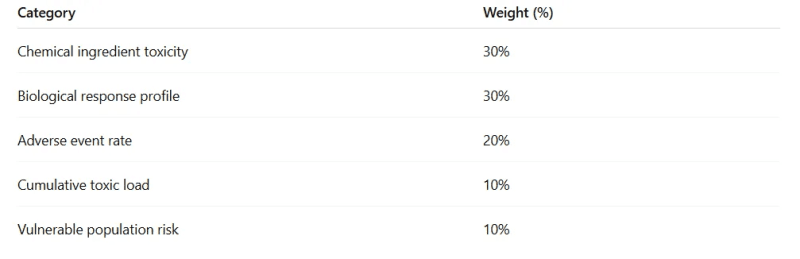

Classification:
- 0–20: Low toxicity
- 21–40: Mild toxicity
- 41–60: Moderate toxicity
- 61–80: High concern
- 81–100: Unacceptable risk (hypothetical)
Conclusion
When it comes to vaccines, the public conversation often stops at a simple phrase: “safe and effective.” Different ingredients have different biological impacts. Reactions can vary by age, genetics, health status, and prior exposures. Without acknowledging these differences, “safe” can mean very different things for different people.
This isn’t about rejecting vaccines. It’s about evolving the conversation from “safe or unsafe” to “how safe, for whom, and under what conditions?” Every medication, from aspirin to chemotherapy, exists on a measurable scale of potential harm and benefit. So why not treat vaccines the same way?
In reality, toxicity is complex. A toxicity index would acknowledge what’s already true: no medical intervention is entirely risk-free.
A toxicity rating system could:
- Enable informed consent through clearer risk communication
- Help identify safer formulations
- Allow customized vaccine choices based on age, risk, or medical history
- Rebuild public trust through transparency
“Safe and effective” may sound reassuring, but science deserves more nuance. A Vaccine Toxicity Index would not undermine vaccines — it would strengthen the science behind them.
Let’s stop pretending risk is binary.
Toxicity isn’t black and white — it’s a spectrum.
Measuring toxicity on a spectrum allows for better science, better policy, and better public understanding.
Republished from the author’s Substack
Join the conversation:


Published under a Creative Commons Attribution 4.0 International License
For reprints, please set the canonical link back to the original Brownstone Institute Article and Author.









