In 2021, Western Australia became the world’s accidental vaccine safety control group. With its closed borders and strict quarantine rules, the ‘Hermit Kingdom’ managed to maintain near-zero Covid while administering almost four million doses of Covid vaccination, resulting in an “exponential increase” in adverse event reports.
In 2022, the Hermit Kingdom control group ended, as community spread finally took off in the leadup to the eventual border opening in March. By May 2022, WA reached the peak of its Covid pandemic, 12-24 months after the rest of the world.
With the recent release of Western Australia’s vaccine safety data for 2022, the chapter closes on this ad-hoc vaccine safety control group. However, new lessons can be drawn from this next chapter in the ongoing global mass vaccination experiment.
IN BRIEF
- Chest pain was the most commonly reported side effect following Covid vaccination
- Young people suffered disproportionately from cardiac injuries
- A dedicated active surveillance program for the Mpox vaccine rollout picked up 52x more adverse events than the passive/active surveillance of all vaccines combined
- Reports of adverse events remained higher than the pre-Covid vaccine average
- Nearly half of those who reported adverse events presented at hospital
- Of 140 deaths reported, 2/3 were determined as not causally associated with vaccination, while 1/3 remain unclassified
KEY INSIGHTS
1. Chest pain was the most commonly reported side effect following Covid vaccination.
The top 10 side effects reported to the West Australian Vaccine Surveillance System (WAVSS) for Covid vaccines included chest pain, pericarditis, shortness of breath, (SOB) and palpitations. Only the Mpox vaccines, which were actively monitored for cardiac symptoms, came close, with SOB, palpitations and chest pain featuring as the fifth, sixth, and seventh most common side effects, respectively.
The report notes that rates of chest pain and pericarditis following Covid vaccination are comparatively over-represented compared to national reporting rates, which is “partially attributable to the data linkage active surveillance processes undertaken by WAVSS.”
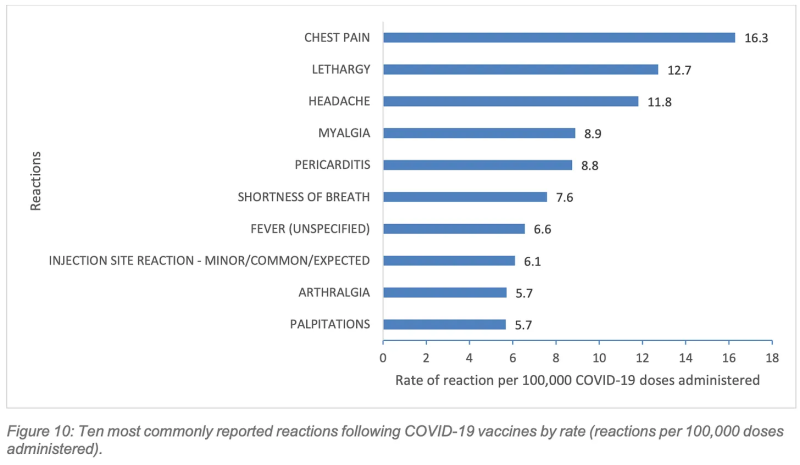
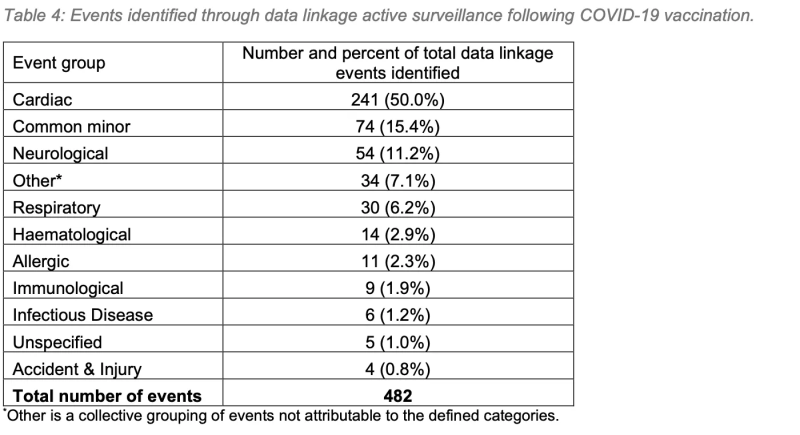
50% of cardiac symptoms following Covid vaccination were identified through data linkage in WAVSS’ active surveillance program. This serves to highlight the importance of active surveillance in identifying adverse events following immunisation (AEFI).
WAVSS confirmed 65 cases of myocarditis/myopericarditis, of which 60 were determined to be causally associated with Covid vaccination. Of 215 confirmed cases of pericarditis, 189 were determined to be causally associated with Covid vaccination, and one with influenza vaccination.
2. Young people suffered disproportionately from cardiac injuries following Covid vaccination, raising serious questions about whether the injections were given with fully informed consent.
Up until mid-to-late 2022, young Australians and their doctors may have been unaware of the true risk:benefit profile of Covid vaccines, thereby precluding the possibility of informed consent. It caused a minor scandal when it was reported, in late 2022, that Australia’s vaccine advisory body, ATAGI, did not know about the cardiac risks to young people until five months after it approved the Covid vaccines for use in this cohort.
In the latter half of 2022, academia caught up with the vaccine rollout, and a series of studies determined that the risks of Covid vaccination outweighed the benefits for healthy children and young adults. For example, a much-circulated study published in the British Medical Journal (BMJ) estimated that 31, 207–42, 836 young adults aged 18–29 years must receive a booster to prevent one Covid hospitalisation over a six-month period.
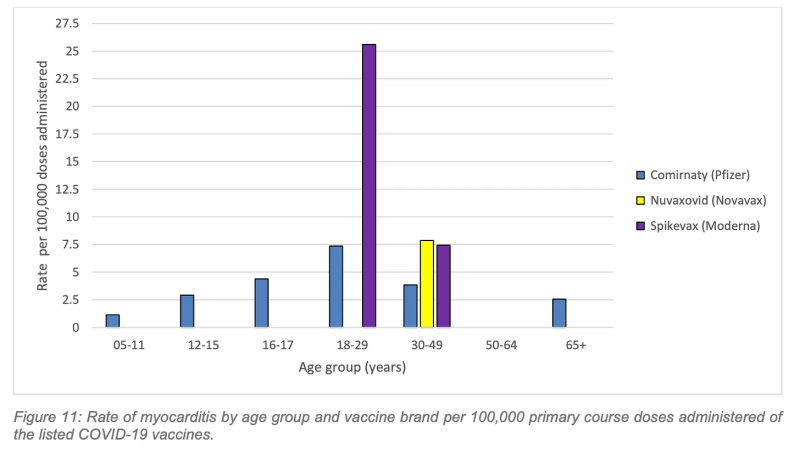
By early 2023, ATAGI updated its booster advice to acknowledge the “comparatively higher risk of myocarditis following vaccination,” but for the young people represented in the graphs below, this advice came too late.
The highest rate of myocarditis following a primary course vaccination was following Spikevax (Moderna) in the 18–29-year age group with a rate of 25.6 cases per 100,000 doses.
The highest rate of myocarditis following a Covid booster was following Spikevax (Moderna) in the 16-17-year age group (Figure 12) with a rate of 41.4 cases per 100,000 Moderna booster doses administered.
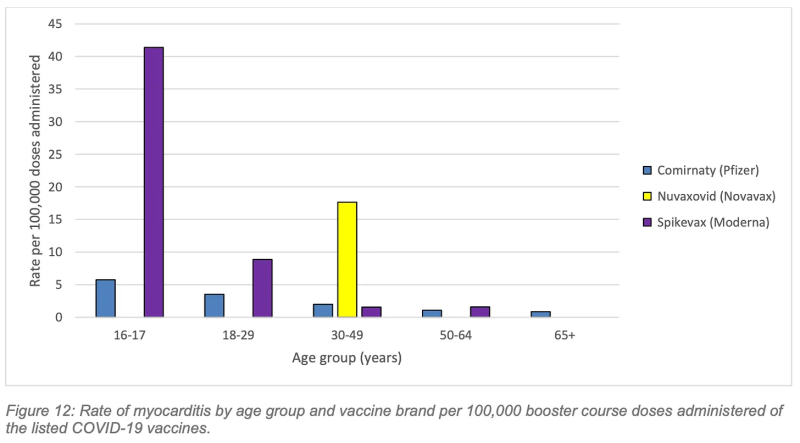
Working to the aforementioned study’s lower estimate of 31, 207 booster vaccinations necessary to prevent one hospitalisation over a six-month period in the 18-29 age cohort, cross-referenced against WA’s 2022 rate of 41.4 myocarditis cases per 100,000 Moderna booster doses (albeit in the 16-17 yo cohort), we’re looking at 12.9 cases of myocarditis inflicted to save one Covid hospitalisation.
The pericarditis rates are worse. The highest rate of pericarditis following a Covid booster was in the 18-29 age group following Nuvaxovid (Novavax), with 55.2 confirmed cases per 100,000 boosters administered.
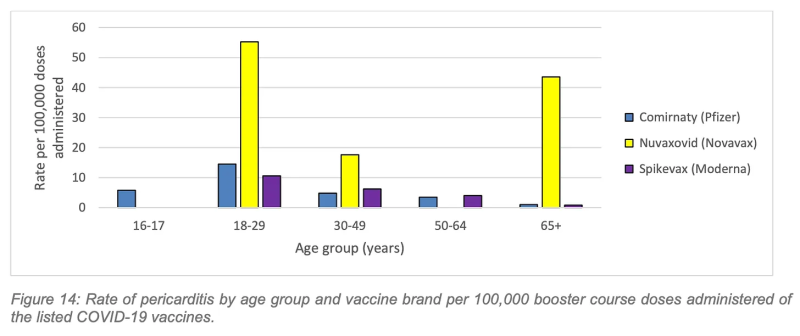
Again, working to the estimate of 31, 207 booster vaccinations necessary to prevent one hospitalisation over a six-month period in the 18-29 age cohort, cross-referenced against WA’s 2022 rate of 55.2 pericarditis cases per 100,000 Novavax booster doses in the same age cohort, we can estimate 17.2 cases of pericarditis inflicted to save one Covid hospitalisation.
3. Active surveillance was successful in identifying 31% of all reported AEFIs, and nearly the totality of reported Mpox AEFIs, highlighting the importance of active surveillance in pharmacovigilance programs.
Active surveillance was successful in identifying 31% of all reported AEFIs, and nearly the totality of reported Mpox AEFIs, highlighting the importance of active surveillance in pharmacovigilance programs.
The active aspect of WA Health’s vaccine safety surveillance in 2022 is commendable, particularly in the monitoring of the Mpox vaccine program, which picked up 52x more adverse events than the passive/active surveillance of all vaccines combined.
From the report,
”Due to the introduction of the new Mpox vaccines and their increased active surveillance, the WAVSS team attempted to contact all patients who reported cardiac symptoms following Mpox vaccination to recommend clinician review or to obtain medical records if they had already been reviewed…”
Data linkage methods were also implemented to investigate co-administration of JYNNEOS and COVID-19 vaccines, which were jointly offered at targeted vaccination clinics. No vaccine safety signals were reported.“
While the overall AEFI reporting rate for vaccines administered in 2022 was 0.6%, the 103 AEFI reports registered in relation to Mpox vaccines resulted in an AEFI reporting rate of 3.3%.
The report states, “The high rate of AEFI reports observed in the Mpox vaccine group illustrates how effective active safety surveillance can be in a closely monitored targeted vaccination program.”
This also highlights the phenomenon of underreporting factor (URF) in passive surveillance (below, red).
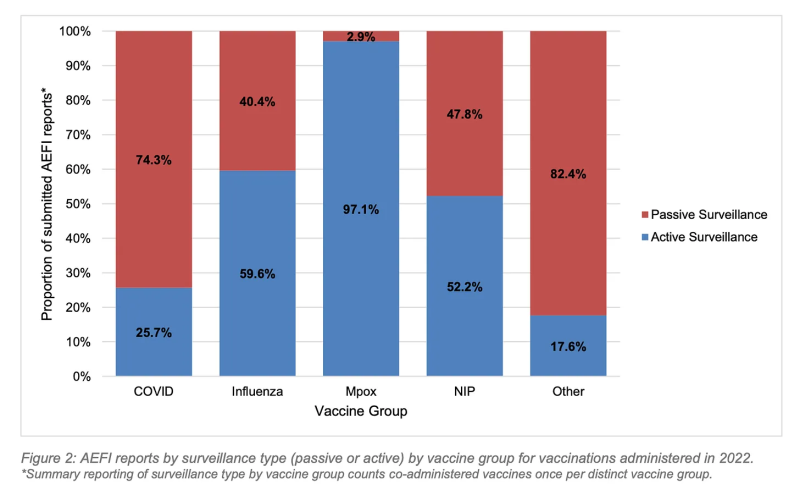
Notably, the MPox active surveillance program was limited to follow-up of patients who were aware of, and reported, cardiac symptoms following vaccination, or who were picked up in data linkage efforts. While this resulted in a much higher AEFI reporting rate than the other vaccines administered in WA in 2022, it presumably would not have picked up subclinical damage.
A Swiss study actively monitoring for subclinical cardiac damage after administration of mRNA Covid boosters found myocardial injury occurred at a rate of 2.8%, or one in every 35 persons. Significantly, not all of these people reported cardiac symptoms or were aware of the damage.
In light of this finding, active surveillance of products with known cardiac side effects could be improved by monitoring subclinical markers.
4. Reports of adverse events remained much higher than the pre-Covid vaccine average.
This was particularly notable in the first quarter, as the jabbing frenzy continued with the introduction of tough proof-of-vaccination rules, ongoing workplace mandates, and the dangled carrot of the border opening in March pending satisfactory booster uptake.
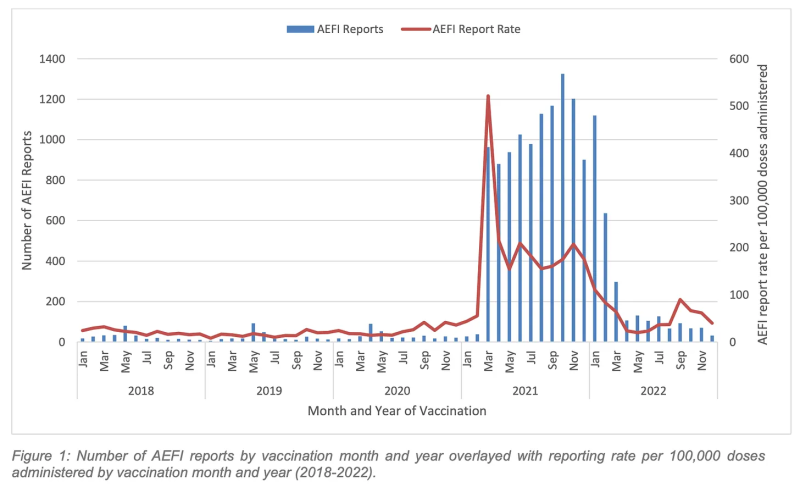
Overall, WA registered 2,853 AEFIs compared with an average of 323 per year for the 2018-2020 (pre-Covid vaccine) period.

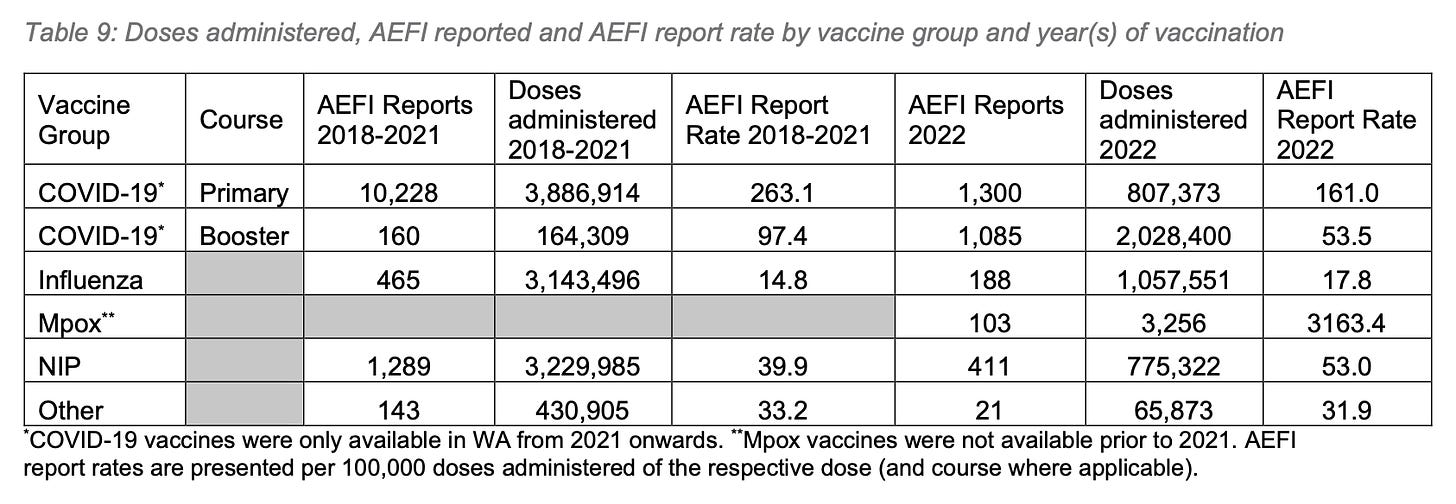
Mostly, this increase was due to the Covid vaccine products, which accounted for 59.9% (2,835,773) of total vaccine doses administered, and were associated with 76.7% (2,385) of reported AEFIs. However, there is a slight increase in the AEFI reporting rate for the National Immunisation Program (NIP), which the report states, “likely reflects the expanding role of active surveillance methods for detecting AEFI, and greater public awareness of the passive surveillance program following the 2021 COVID-19 vaccine roll-out.”
The AEFI reporting rate from the total 4,737,775 vaccine doses administered was 60.2 per 100,00 doses, or 0.6%. For all Covid products combined, the AEFI reporting rate was 84.1 per 100,000 doses, which, curiously, is lower than the national reporting rate of 200 per 100,000 doses since the beginning of the rollout.
The report offers as explanation:
”This figure is likely a reflection of the increased proportion of booster doses administered in 2022, which is consistent with international data demonstrating lower AEFI rates following booster doses as compared to primary course doses.”
The WAVSS data appear to support this hypothesis. The primary series Covid vaccines yielded an AEFI reporting rate of 161 per 100,000 doses, 3x the booster dose AEFI reporting rate of 53.5 per 100,000 doses administered.
The report adds,
”Additionally, this figure also reflects a reduction in reporting of common, minor AEFI, likely due to increased public awareness of these AEFI.”
This statement implies the 2022 report may document a larger proportion of serious AEFIs than the 2021 report.
5. Nearly half of those who reported AEFIs presented at hospital
Thirty-seven percent of those who registered AEFIs were dealt with in the emergency department (ED), while 11% were admitted to hospital, totalling 48%. This is slightly down on last year’s figures, but still high considering the dominant media and public health messaging that most side effects are mild.

6. Of 140 deaths reported, 2/3 were determined not causally associated with vaccination, while 1/3 remain unclassified.
One-hundred thirty of the 140 registered deaths following vaccination were identified via active surveillance of records searching for deaths occurring within 21 days of vaccination.
The report states that all cases received specialist review, but only 86 cases were determined to have information sufficient to rule out a causal association. 44 cases remain unclassified, as confirmation of the final cause of death from a death certificate or coroner report is pending.
The report states, “There is no indication that these deaths are likely to be attributed to vaccination based on initial investigation.” However, there appears to be no evidence that the deaths are not causally associated with vaccination, either.
WA HEALTH RESPONDS
WA Health was asked to comment on what it considers to be the benchmark rate of serious AEFIs, by age group, to halt a vaccine program. This question is pertinent because the Covid vaccine program has far surpassed the rate of serious AEFIs reported from the Fluvax Junior program. The Fluvax Junior program was halted after WA Health collaborated with the Therapeutic Goods Administration (TGA) in identifying a spate of reported serious AEFIs, leading to an investigation and official recognition of a causal link between the vaccine product and the AEFIs.
A spokesperson for the WA Health replied,
“Safety of medical interventions, including vaccinations, is assessed by simultaneously considering the likely risks compared to the likely benefits. The TGA is the federal authority ultimately responsible for ongoing assessment of the safety of medicines registered for use in Australia, including vaccines.”
In other words, WA Health oversees the ‘bean counting’ aspect of vaccine safety surveillance (collected and collated by WAVSS), but does not make any assessments about the safety of the corresponding medical interventions.
WA Health was also asked whether autopsies were performed as part of the “specialist review” determining the causality of reported deaths after vaccination.
The reply,
”Where possible, cause of death information is sourced from the WA Death Registry. It is not routinely reported as to whether a person underwent autopsy prior to having their cause of death recorded on the WA death registry.”
Given the high rate of causality identified in deaths after Covid vaccination when autopsies are performed, it is problematic that the Department cannot confirm if autopsies were conducted to establish the cause of death for the 140 cases in the 2022 report.
CONCLUSIONS
The Western Australian Vaccine Safety Surveillance Report 2022 demonstrates the importance of undertaking active surveillance in monitoring new vaccine rollouts.
The active surveillance effort undertaken by WAVSS and its partner systems is commendable, and, despite some questionable editorialising, the resultant report provides a thorough summary of WA’s vaccine safety surveillance during 2022.
The cardiac damage inflicted on WA’s young people for such little benefit should serve as a sobering warning to health professionals to ensure that informed consent and the precautionary principle remain front and centre in their practice.
Finally, the sustained high rates in adverse event reporting following vaccination since the Covid vaccine rollout, many of which have been serious enough to require management in emergency departments and hospitals, indicates an urgent need for a review of the benchmark indicators for withdrawal of a product from the market until safety can be guaranteed.
Republished from the author’s Substack
Published under a Creative Commons Attribution 4.0 International License
For reprints, please set the canonical link back to the original Brownstone Institute Article and Author.









