In addition to planning a new rollout of the Covid-19 vaccination campaign in the fall, the European Union is already reserving “ever warm” novel vaccine production capacity for the next public health emergency: this as part of a so-called EU FAB initiative under the aegis of the EU’s recently-created Health Emergency Preparedness and Response Authority (HERA).
The public tender was announced by the European Commission in the same April 27th documents in which it announced its intention to target the still unvaccinated and children for Covid-19 vaccination in the fall.
The Commission press release explains that the purpose of the tender is:
…to reserve capacities for manufacturing mRNA, protein and vector-based vaccines. This will reserve newly created manufacturing capacity for use in future health emergencies. The tender is addressed to vaccine producers with facilities in the EU/EEA, who can send in their request to participate until 3 June 2022 16.00 CEST.
The tender is available here, and a fact sheet here. A prior information notice on the “Establishment of a Network of Ever-warm Production Capacities for Vaccines and Therapeutics manufacturing (EU FAB)” was already published last September.
The tender announcement and related documents mention three different sorts of novel vaccines: mRNA, protein, and vector-based. But in light of the EU’s Covid-19 response, it is clear that the actual emphasis is likely to be placed on mRNA.
Although the Astra-Zeneca and Johnson and Johnson viral vector vaccines were part of the EU’s initial Covid-19 vaccine rollout in winter 2020/2021, their use has been de facto discontinued for nearly a year now.
By contrast, the European Commission’s initial order of 600 million doses of the BioNTech-Pfizer mRNA vaccine (as documented here) has since burgeoned to a total of 2.4 billion doses (as can be seen here). The Moderna mRNA vaccine also continues to be used in the EU, but far less than BioNTech-Pfizer.
The below “Our World in Data” graph illustrates this predominance of the mRNA vaccines, and especially the BioNTech-Pfizer vaccine, in the EU.
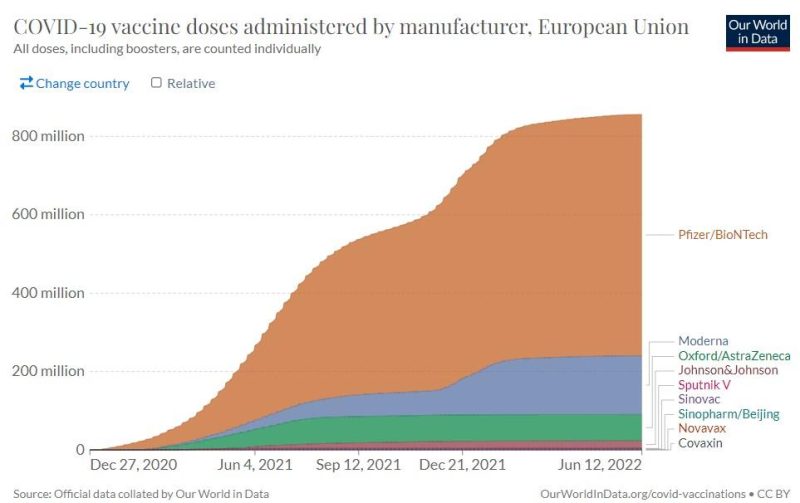
Last December, the European Medicines Agency also authorized the use of the Novavax protein-based vaccine. But as the above graph likewise makes clear, Novavax has barely made a dent in the EU market. (Many of the vaccines listed above it are not even authorized by the EU, but only in individual members states.)
This is hardly surprising, since the EMA has only authorized its use for primary immunization, not as a booster, and, according to official statistics, nearly 85% of adults in the EU have already been vaccinated.
The EU FAB announcement’s reference to reserving “newly created manufacturing capacity” is perhaps an allusion to BioNTech’s 2020 purchase of the Behringwerke production facility in Marburg. Unlike its commercial partner Pfizer, which markets its vaccine in most of the Western world, BioNTech did not in fact have any manufacturing capacity prior to its acquisition of the Behringwerke, since before the authorization of its Covid-19 vaccine it had never brought a product to market.
The EU FAB tender comes on the heels of – and is clearly modeled on – a German tender of exactly the same sort, which resulted in the German government concluding “pandemic preparedness contracts” with five suppliers in April. All five are German and all five are involved in the development of novel vaccines.
They are: BioNTech – here without its American partner Pfizer – Curevac in partnership with GlaxoSmithKline, a German/German partnership of Wacker and CordenPharma, Celonic, and IDT Dessau. Curevac, another would-be mRNA vaccine manufacturer, was also a player in the “race” to create a Covid-19 vaccine. But, apart from BioNTech and Curevac, most readers will probably not have heard of the others.
Most German readers will not have heard of them either. As the ÄrzteZeitung, a specialized German newspaper for MDs, notes: “of the suppliers, only BioNTech (Comirnaty®) … has a product on the market to date.” And BioNTech’s vaccine, we might add, is still only on the market in Europe under a “conditional,” i.e. emergency, authorization.
Under the contracts, the German government will pay the suppliers to reserve capacity for producing up to (or, in the case of BioNTech, at least) 80 million doses of hitherto unspecified vaccines per year. The aim, per a March press release of the German Ministry of Health, is to ensure German government access to their capacity “ in the case of the persistence of the Covid-19 pandemic or a new pandemic.”
Furthermore, the ministry press release notes that the contracts will help to ensure “German supply of vaccines from its own production.” This is a somewhat odd goal in light of the fact that EU member states in general have been required to receive their supply of Covid-19 vaccines via contracts centrally negotiated by the European Commission, which, as noted above, has procured the bulk of the vaccine supply precisely from the BioNTech-Pfizer partnership.
The goal of German vaccine autarky is also strangely at odds with the EU’s stated aim of creating a “European Health Union,” of which the Health Emergency Preparedness and Response Authority is supposed to be a “main pillar.” The German ministry describes the German contracts as “internationally expandable,” suggesting that some of these German companies will also figure among the recipients of the EU-level contracts.
Published under a Creative Commons Attribution 4.0 International License
For reprints, please set the canonical link back to the original Brownstone Institute Article and Author.